Glial Cell Communities Conspire to Drive Alzheimer’s Disease
Quick Links
The amyloid cascade—plaques to tangles to cognitive decline—has become well established. Less well understood is what role inflammation and glial changes play. Now, by analyzing single-nuclei RNA-Seq data from more than 400 postmortem brains, scientists have homed in on specific glial subtypes associated with distinct stages of AD pathology, estimating the effects subtypes have on plaques, tangles, and cognition. The study, led by Philip De Jager and Vilas Menon at Columbia University Irving Medical Center in New York, David Bennett at Rush University Medical Center, Chicago, and Naomi Habib at The Hebrew University of Jerusalem, helps refine the amyloid cascade, filling in the role of inflammation. It was published in the September Nature. “Alzheimer’s is not a single-cell disease, but a disease of the whole brain environment,” De Jager told Alzforum.
- Single-nucleus RNA-Seq spots three glial subtypes that contribute to AD pathology.
- One type of microglia promotes plaques, another tangles.
- An astrocyte subtype provoked cognitive decline.
- Yet other glia seemed to improve resilience to plaques.
This supports the idea, articulated by Bart de Strooper and others, that toxic forms of Aβ and tau precipitate a cellular phase of AD (Mar 2016 webinar).
Notably, while in some plaque-ridden brains the glial changes seemed to promote tangles and cognitive decline, in others, cell subtypes took an alternative trajectory. This was characterized by stable plaques, few tangles, and less memory loss, potentially representing a more resilient response to amyloid. Some of this work was previously reported at the 2022 Alzheimer’s Association International Conference (Sep 2022 conference news; Sep 2022 conference news).
Other researchers were enthusiastic. “This is a fascinating and timely study that deepens our understanding of cellular dynamics in aging and Alzheimer’s disease,” Jonathan Kipnis at Washington University, St. Louis, wrote to Alzforum. Brad Friedman at Genentech, South San Francisco, called it a significant achievement, noting that its size allowed it to define more cellular subtypes than had previous work (comments below).
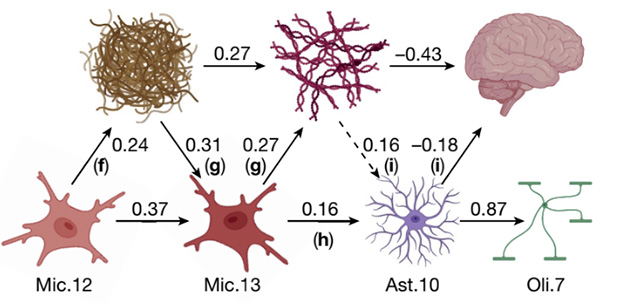
Driving the Amyloid Cascade? A microglial subtype, Mic.12, promotes plaque deposition, while another, Mic.13, promotes tangles. An astrocyte subtype, Ast.10, contributes to cognitive decline, as well as perturbing oligodendrocytes. Numbers indicate the strength of each effect. [Courtesy of Green et al., Nature.]
Panoply of Cells
Several earlier single-nuclei RNA-Seq studies had parsed glial cells into numerous activation states (May 2019 news; Sep 2022 conference news; Oct 2023 news). However, because such studies are cross-sectional and descriptive, they offered little insight into how these states related to each other and how they changed over time.
To gain a more nuanced view of the aging brain, the authors leveraged data from the Religious Order Study and Rush Memory and Aging Project (ROSMAP), an observational cohort where participants start out cognitively healthy and consent to brain donation after death. The authors analyzed 437 brains. The donors’ average age at death was 89, and two-thirds were women. Cognitive status at the time of death spanned the gamut, with 38 percent cognitively healthy, 26 percent mildly cognitively impaired, and 36 percent with AD dementia. Alzheimer’s pathology was common even in unimpaired participants, with most brains at Braak stage III, IV, or V. Almost two-thirds of the participants met the pathological criteria for AD.
First author Gilad Green isolated more than 1.6 million nuclei from dorsolateral prefrontal cortex samples for RNA-Seq. The transcriptomes represented all major brain cell types, and clustered into 95 distinct cellular subtypes, including 16 microglia, 10 astrocytes, and 12 oligodendrocytes. To link these cell states to AD, Green and colleagues tested their association with neocortical plaque burden and tangle density, as well as with the rate of cognitive decline measured in annual tests. Then they used mediation analysis, which estimates how much a given factor contributes to a downstream event, to infer causal associations.
Kyle Travaglini at the Allen Institute for Brain Science, Seattle, noted that RNA-Seq studies are typically limited by their lack of functional data. “Green et al.’s big advance is applying mediation modeling to overcome this,” he wrote (comment below).
Fitting Glia into the Amyloid Cascade
The authors found that in older brains a subset of microglia tended to assume a reactive, amoeboid state, characterized by perturbed lipid metabolism and the expression of endocytosis and phagocytosis genes. These microglia, dubbed Mic.12, promoted amyloid deposition. Plaques then helped prod these microglia into a new state, Mic.13. These poorly metabolized lipids, and ramped up expression of many extracellular matrix and microglial AD genes, such as TREM2, ApoE, SPP1, and GPNMB. Intriguingly, in brains of APOE4 carriers, Mic.13 were more likely to emerge.
Mic.13 helped spark tau pathology, mediating a third of the association between plaques and tangles. Mic.13 and tangles together triggered a reactive astrocyte state, Ast.10, characterized by expression of oxidative stress genes. Tangles and Ast.10 both contributed to cognitive decline. Ast.10 also goaded oligodendrocytes into a new state, Oli.7, with weakened cholesterol synthesis and more expression of stress response genes (image above).
Other studies have described similar microglial states in AD brain (Gerrits et al., 2021). “The disease-associated subpopulations of microglia that [the authors] define are molecularly consistent with definitions in our recent meta-analysis,” noted Evan Macosko at the Broad Institute of MIT and Harvard (Jul 2023 news).
Previous work has likewise implicated astrocytes in the amyloid cascade (Jun 2023 news). Curiously, in the ROSMAP cohort, Ast.10 also contributed to cognitive decline that was not associated with plaques or tangles, hinting that this astrocyte state might help explain other neurodegenerative conditions as well.
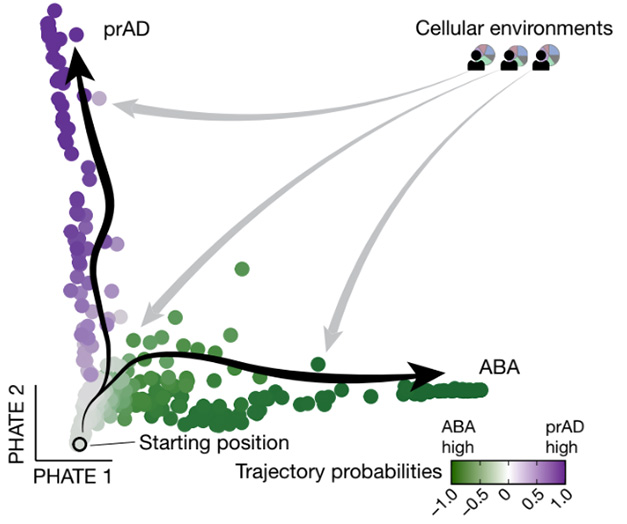
Two Ways to Age. Healthy, plaque-free brains have homeostatic glia (gray). In brains with plaques, glia assume distinct reactive states during progression to (prAD) or during alternative brain aging (ABA). [Courtesy of Green et al., Nature.]
Going BEYOND
To relate these findings to aging, the authors developed an algorithm, BEYOND, that compared abundances of each cellular subtype across all 437 brains from the ROSMAP cohort, and used small differences in those numbers to order the brains into hypothetical aging trajectories. BEYOND identified two distinct trajectories among people who had brain amyloid. One, dubbed progression to AD, followed the path described above, with prominent roles for Mic.12, Mic.13, and Ast.10 (image above). The second, designated alternative brain aging (ABA), involved a growth in other cell populations, such as Ast.5, which had a similar profile to mouse disease-associated astrocytes, and the oligodendrocyte precursor population OPC.3, which associated with axon regeneration (Habib et al., 2020). “The endpoints of each trajectory are driven by different subtypes of astrocytes,” De Jager noted.
Some people along the ABA pathway did have dementia. Therefore, De Jager does not believe it represents healthy aging. Healthy agers were more likely those people in the cohort who followed neither trajectory, but instead maintained their glia in a homeostatic state, he noted. However, the relatively stable plaque level, lack of tangles, and slower cognitive decline in ABA could indicate some form of resilience to amyloid.
In future work, the authors will use spatial transcriptomics to see if the glial subtypes they found occur together and interact directly. They will also study the Ast.10 subtype in vitro to find out what triggers it and how to prevent its formation. “Averting the polarization of microglia and astrocytes into Mic.13 and Ast.10, respectively, opens new routes for interventions into preventing the symptomatic manifestations of AD,” they suggested.—Madolyn Bowman Rogers
References
Webinar Citations
News Citations
- RNA-Seq from 2.8 Million Cells Yields New Clues About Alzheimer's
- Pseudotime Simulates Disease Progression from Case-Control RNA-Seq
- When It Comes to Alzheimer’s Disease, Do Human Microglia Even Give a DAM?
- Meta-Analysis of RNA-Seq Data is Robust, Finds Key Changes in AD
- Stunning Detail: Single-Cell Studies Chart Genomic Architecture of AD
- Cortical Biopsies Hint at Start of Alzheimer's 'Cellular Phase'
- In Amyloid Cascade, Do Reactive Astrocytes Bridge Plaques and Tangles?
Paper Citations
- Gerrits E, Brouwer N, Kooistra SM, Woodbury ME, Vermeiren Y, Lambourne M, Mulder J, Kummer M, Möller T, Biber K, Dunnen WF, De Deyn PP, Eggen BJ, Boddeke EW. Distinct amyloid-β and tau-associated microglia profiles in Alzheimer's disease. Acta Neuropathol. 2021 May;141(5):681-696. Epub 2021 Feb 20 PubMed.
- Habib N, McCabe C, Medina S, Varshavsky M, Kitsberg D, Dvir-Szternfeld R, Green G, Dionne D, Nguyen L, Marshall JL, Chen F, Zhang F, Kaplan T, Regev A, Schwartz M. Disease-associated astrocytes in Alzheimer's disease and aging. Nat Neurosci. 2020 Jun;23(6):701-706. Epub 2020 Apr 27 PubMed.
Further Reading
News
- Transcriptomics Paint Astrocytes as Source of Cognitive Resilience
- At the Edge of Spatial Omics, Cellular Response to Amyloid Comes into View
- Two Paths for TREM2-Positive Microglia: DAM or Senescence?
- Tauopathy Transcriptomes Tell Tantalizing Tales
- Transcriptomics Confirm Vascular Changes in Alzheimer’s Brain
- Higher-Resolution Spatial Transcriptomics Maps Mayhem Near Plaques
Primary Papers
- Green GS, Fujita M, Yang HS, Taga M, Cain A, McCabe C, Comandante-Lou N, White CC, Schmidtner AK, Zeng L, Sigalov A, Wang Y, Regev A, Klein HU, Menon V, Bennett DA, Habib N, De Jager PL. Cellular communities reveal trajectories of brain ageing and Alzheimer's disease. Nature. 2024 Sep;633(8030):634-645. Epub 2024 Aug 28 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.