Tauopathy Transcriptomes Tell Tantalizing Tales
Quick Links
Fibrillar tau accumulates in several neurodegenerative diseases. Are the same molecular mechanisms responsible, or does each tauopathy follow distinct pathways? Some of both, according to a preprint posted to bioRxiv on September 30. Researchers led by Jessica Rexach and Daniel Geschwind at the University of California, Los Angeles, compared single-nuclei RNA-Seq of postmortem tissue from people who had died with Alzheimer’s disease, progressive supranuclear palsy, or behavioral variant frontotemporal dementia. PSP and bvFTD are primary tauopathies, whereas in AD, tau pathology is secondary to Aβ. Though snRNA-Seq studies have blossomed in recent years, this is the first study to directly compare the three disorders.
- AD, behavioral variant FTD, and PSP have more similarities than differences.
- However, while AD risk genes are typically expressed in microglia, bvFTD and PSP genes emerge as neuronal.
- In PSP, astrocytes are especially affected, and express lots of tau.
The tauopathies were more alike than different. Ninety-five percent of genes that were differentially expressed in tauopathies compared to age-matched control brains were similarly altered in all three diseases.
Nonetheless, there were differences that help explain the etiology of these distinct disorders. Most AD risk genes were expressed in microglia, as previous studies have found, but in the primary tauopathies, most risk genes were found in neuronal subtypes. Glia played different roles in primary tauopathies, too. For example, astrocytes were particularly vulnerable in PSP, a disorder in which tau deposits accumulate in astrocytes. Many differences were specific to a particular brain region, highlighting the importance of studying multiple areas. Rexach had reported some of these data at the Tau2022 conference (Mar 2022 conference news).
Scientists praised the study. “An understanding of the molecular mechanisms that are common and distinct in different tauopathies is of great interest and therapeutic significance,” said Martin Kampmann at the University of California, San Francisco. Rik van der Kant at Vrije University, Amsterdam, noted that much less is known about cellular changes in PSP and FTD than in AD, where there have been numerous snRNA-Seq studies (e.g., Oct 2023 news). “This manuscript is therefore a milestone for these diseases and a treasure trove for the field,” he wrote to Alzforum (comments below).
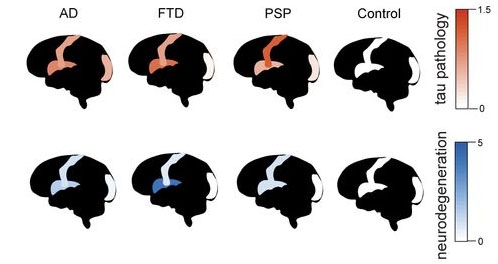
Representative Regions. Tau pathology (red) is highest in the insular cortex (middle of brain) in FTD, and in primary motor cortex (stripe) in PSP. Neurodegeneration (blue) follows these patterns. The visual cortex (back of brain) is largely spared in all three disorders. [Courtesy of Rexach et al., bioRxiv.]
Universal Features
First author Rexach collaborated with scientists at UCSF and the University of Pennsylvania to analyze tissue from their respective brain banks. The study used 40 brains, 10 each for the three tauopathies and 10 age-matched controls. The authors chose to focus on three cortical brain regions: insula, primary motor, and visual. Insular cortex is particularly affected in bvFTD, and primary motor in both PSP and AD. Visual cortex is largely spared in all three disorders, allowing the scientists to study resilience mechanisms (image above). Altogether, the authors analyzed 590,541 cells.
Perhaps the most notable common change occurred in a subtype of inhibitory interneuron in the insular cortex. This recently identified cell type occupies white matter, and is characterized by expression of the transcription factor MEIS2 and the protease ADAMTS19 (Bakken et al., 2021; BRAIN Initiative Cell Census Network, 2021; Oct 2023 news). In all three tauopathies, these neurons showed signs of activating a cellular stress response, amping up genes responsible for protein folding, while turning down DNA repair genes. “That population has not been implicated [in disease] before,” Rexach noted.
Meanwhile, the transcription factor RORB, which represses protective synaptic and cellular stress response genes, marked excitatory insular neurons that were vulnerable to cell death in both AD and bvFTD. Scientists led by Kampmann and Lea Grinberg at UCSF had previously flagged this gene as a marker of neurons particularly prone to neurofibrillary tangles in AD, but its role in bvFTD was unknown (Jan 2021 news). The authors also found shared glial changes, including an increase in activated microglia, and less expression of injury response genes in oligodendrocyte precursor cells.
Some shared changes were protective. In the insular cortex of all three diseases, excitatory neurons that expressed the transcription factors MAFG and NFE2L1 had fewer tau deposits. These genes promote autophagy, iron sequestration, and a protective antioxidant response. They also boost VCP expression, which is known to lower tau seeding (Zhu et al., 2022).
Divergent Features
What about disease-specific changes? In AD motor cortex, the authors found an abundance of a microglial subtype previously reported to be associated with plaques (Jul 2020 news). These microglia expressed the familial dementia gene ITM2B, as well as many other AD risk genes.
In bvFTD insular cortex, large numbers of layer 2/3 excitatory neurons expressed FTD/ALS genes involved in autophagy and the stress response. At the same time, a different layer 2/3 excitatory subtype, distinguished by transcription factor CUX2 and synaptic gene CBLN2, was in short supply. This cortical layer carries the highest tau burden in bvFTD.
In PSP insular cortex, the authors discovered a shortage of certain layer 5/6 excitatory neurons that expressed many PSP risk genes. These projected to subcortical structures, such as the striatum, that accumulate high tangle burden in PSP.
PSP astrocytes seemed under particular duress. In the visual cortex, they expressed less REST than in control brain; this transcription factor has been shown to maintain astrocyte identity (Masserdotti et al., 2015). Throughout PSP brain, astrocytes highly expressed the tau gene MAPT, while turning down genes responsible for tau degradation. “This is a very important finding, as it may explain why tau pathology occurs in astrocytes in PSP patients,” van der Kant noted.
Van der Kant was also intrigued by an increase in cholesterol metabolism in PSP neurons, as he had found the same thing occurs in AD (Feb 2019 news). “These new data … indicate that brain-cholesterol targeting interventions such as low-dose efavirenz might be clinically relevant for PSP as well,” he wrote. Van der Kant discovered that efavirenz, an FDA-approved medication for HIV infection, triggers cholesterol degradation—a potential therapy for AD. The drug is being tested in a Phase 1 trial in Cleveland.
Rexach and colleagues will make their data available to other scientists once it is peer-reviewed and published.—Madolyn Bowman Rogers
References
News Citations
- Tau Triggers Neuroinflammation, But Mechanisms Vary by Disease
- Stunning Detail: Single-Cell Studies Chart Genomic Architecture of AD
- Behold, the Human Brain Like Never Seen Before
- Selective Vulnerability News: RORB Neurons Are First Victims of Tangles
- Paper Alert: Those PIGs! Spatial Transcriptomics Add Human Data
- Cholesteryl Esters Hobble Proteasomes, Increase p-Tau
Therapeutics Citations
Paper Citations
- Bakken TE, Jorstad NL, Hu Q, Lake BB, Tian W, Kalmbach BE, Crow M, Hodge RD, Krienen FM, Sorensen SA, Eggermont J, Yao Z, Aevermann BD, Aldridge AI, Bartlett A, Bertagnolli D, Casper T, Castanon RG, Crichton K, Daigle TL, Dalley R, Dee N, Dembrow N, Diep D, Ding SL, Dong W, Fang R, Fischer S, Goldman M, Goldy J, Graybuck LT, Herb BR, Hou X, Kancherla J, Kroll M, Lathia K, van Lew B, Li YE, Liu CS, Liu H, Lucero JD, Mahurkar A, McMillen D, Miller JA, Moussa M, Nery JR, Nicovich PR, Niu SY, Orvis J, Osteen JK, Owen S, Palmer CR, Pham T, Plongthongkum N, Poirion O, Reed NM, Rimorin C, Rivkin A, Romanow WJ, Sedeño-Cortés AE, Siletti K, Somasundaram S, Sulc J, Tieu M, Torkelson A, Tung H, Wang X, Xie F, Yanny AM, Zhang R, Ament SA, Behrens MM, Bravo HC, Chun J, Dobin A, Gillis J, Hertzano R, Hof PR, Höllt T, Horwitz GD, Keene CD, Kharchenko PV, Ko AL, Lelieveldt BP, Luo C, Mukamel EA, Pinto-Duarte A, Preissl S, Regev A, Ren B, Scheuermann RH, Smith K, Spain WJ, White OR, Koch C, Hawrylycz M, Tasic B, Macosko EZ, McCarroll SA, Ting JT, Zeng H, Zhang K, Feng G, Ecker JR, Linnarsson S, Lein ES. Comparative cellular analysis of motor cortex in human, marmoset and mouse. Nature. 2021 Oct;598(7879):111-119. Epub 2021 Oct 6 PubMed.
- BRAIN Initiative Cell Census Network (BICCN). A multimodal cell census and atlas of the mammalian primary motor cortex. Nature. 2021 Oct;598(7879):86-102. Epub 2021 Oct 6 PubMed.
- Zhu J, Pittman S, Dhavale D, French R, Patterson JN, Kaleelurrrahuman MS, Sun Y, Vaquer-Alicea J, Maggiore G, Clemen CS, Buscher WJ, Bieschke J, Kotzbauer P, Ayala Y, Diamond MI, Davis AA, Weihl C. VCP suppresses proteopathic seeding in neurons. Mol Neurodegener. 2022 Apr 12;17(1):30. PubMed.
- Masserdotti G, Gillotin S, Sutor B, Drechsel D, Irmler M, Jørgensen HF, Sass S, Theis FJ, Beckers J, Berninger B, Guillemot F, Götz M. Transcriptional Mechanisms of Proneural Factors and REST in Regulating Neuronal Reprogramming of Astrocytes. Cell Stem Cell. 2015 Jul 2;17(1):74-88. Epub 2015 Jun 25 PubMed.
External Citations
Further Reading
News
- Transcriptomics Confirm Vascular Changes in Alzheimer’s Brain
- New Stem Cell-Derived Neuron Datasets Available
- Single-Nuclei Multi-omics Spots Wonky Gene Regulation
- Higher-Resolution Spatial Transcriptomics Maps Mayhem Near Plaques
- ALS/FTD Genes Converge on Endolysosomal System, Stoking TDP-43 Pathology
- Meta-Analysis of RNA-Seq Data is Robust, Finds Key Changes in AD
- RNA-Seq from 2.8 Million Cells Yields New Clues About Alzheimer's
- ADAD and LOAD: At Cellular Level, They Are Not the Same
- Single-Cell Transcription Cum Chromatin Analysis Pins SREBF1 to AD
- International Symposium Puts PSP/CBD on the Map
- Rogue Gene Networks Track with Neurodegeneration Across Diseases
- Microglia in Tauopathy: Not Just Homeostatic Versus DAM
Primary Papers
- Rexach JE, Cheng Y, Chen L, Polioudakis D, Lin LC, Mitri V, Elkins A, Yin A, Calini D, Kawaguchi R, Ou J, Huang J, Williams C, Robinson J, Gaus SE, Spina S, Lee EB, Grinberg LT, Vinters H, Trojanowski JQ, Seeley WW, Malhotra D, Geschwind DH. Disease-specific selective vulnerability and neuroimmune pathways in dementia revealed by single cell genomics. bioRxiv. 2023 Sep 30; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of California, San Francisco
Tau pathology is a common feature of several neurodegenerative diseases, but there are intriguing disease-specific differences: Each tauopathy is associated with a different spectrum of genetic risk factors, distinct cell types are affected, and recent cryo-EM structures have found different tau fibril conformations associated with different diseases. Therefore, an understanding of the molecular mechanisms that are common and distinct in different tauopathies is of great interest and therapeutic significance.
The new single-nucleus RNA sequencing and ATAC sequencing study by Jessica Rexach, Dan Geschwind and colleagues deeply profiles brain cells from patients with Alzheimer’s disease (AD), behavioral variant frontotemporal dementia (bvFTD), and progressive supranuclear palsy (PSP). Three brain regions with differential vulnerability to the different tauopathies were sampled and systematically compared.
Differentially expressed genes shared many commonalities across diseases, but AD microglia showed disease-specific signatures, which were associated with AD risk genes.
Intriguing new findings include the molecular description of selectively vulnerable neurons in PSP, which differ from previously described selectively vulnerable neuronal subtypes in AD and bvFTD. PSP-vulnerable neurons were enriched for the expression of several genes associated with PSP risk—recapitulating a theme previously observed in other diseases, such as Parkinson’s.
RORB, a marker previously described by us (Leng et al., 2021), was found to also mark vulnerable neurons in bvFTD. Rexach and colleagues map RORB targets that are differentially expressed in vulnerable neurons, which include the RORB-repressed synapse homeostasis gene NPTX2 and a cluster of stress response genes.
Furthermore, the authors found a decreased frequency of astrocytes in PSP, which could be due to cell death, or a change in cellular identity. The latter model is supported by the finding that astrocytes in PSP ectopically expressed neuronal transcription factors.
References:
Leng K, Li E, Eser R, Piergies A, Sit R, Tan M, Neff N, Li SH, Rodriguez RD, Suemoto CK, Leite RE, Ehrenberg AJ, Pasqualucci CA, Seeley WW, Spina S, Heinsen H, Grinberg LT, Kampmann M. Molecular characterization of selectively vulnerable neurons in Alzheimer's disease. Nat Neurosci. 2021 Feb;24(2):276-287. Epub 2021 Jan 11 PubMed.
Vrije Universiteit Amsterdam
This manuscript is enormously important for understanding disease mechanisms in PSP and FTD. In Alzheimer’s, the identification of many genetic risk variants over the last decade had shown us the crucial involvement of lipid metabolism, immune dysfunction, and the endosomal system in this disease. Conversely, beyond the known tau variants themselves, we know very little about which biological processes contribute to disease in the much rarer pure tauopathies such as FTD and PSP. Similarly, single-nuclear RNA-Seq studies have helped tremendously to better understand AD, but similar studies for FTD and PSP are rare. This manuscript is therefore a milestone for these diseases and a treasure trove for the field.
Many interesting conclusions can be drawn from the data here. What I find most novel, and intriguing, is the discovery of unknown disease-specific pathways and vulnerabilities. In PSP, the authors discover a reduction in astrocyte number, a dysregulation of the astrocytic REST complex that controls astrocytic identify, and an upregulation of MAPT expression. This is a very important finding, as it may explain why tau pathology occurs in astrocytes in PSP patients.
In addition, cholesterol biosynthesis was among the significantly changed pathways in neurons in PSP patients. Even as a lipid aficionado, I had not expected that. In Alzheimer iPSC neurons, we have previously shown that cholesterol is a major regulator of tau levels, but in AD a link with cholesterol-transporting genes, such as ApoE, is easily made (van der Kant et al., 2019). This new data strengthens a link between cholesterol and tau also in pure tauopathies, and indicates that brain-cholesterol targeting interventions, such as low-dose efavirenz, might be clinically relevant for PSP as well (van der Kant et al., 2019).
The authors also implicate WNT signaling and synaptic vesicle cycles as major changed pathways in PSP, which should encourage much research into this novel direction.
It will be exciting to if see future functional studies validate the many findings in this paper. It is encouraging that the authors identify many pathways already known to be involved in AD (microglial changes) and in selective vulnerability (RORB). For FTD and PSP many of the findings are new, and I believe for these diseases this paper is a leap forward to understanding the underlying biology.
References:
van der Kant R, Langness VF, Herrera CM, Williams DA, Fong LK, Leestemaker Y, Steenvoorden E, Rynearson KD, Brouwers JF, Helms JB, Ovaa H, Giera M, Wagner SL, Bang AG, Goldstein LS. Cholesterol Metabolism Is a Druggable Axis that Independently Regulates Tau and Amyloid-β in iPSC-Derived Alzheimer's Disease Neurons. Cell Stem Cell. 2019 Mar 7;24(3):363-375.e9. Epub 2019 Jan 24 PubMed.
University of Iowa
This intriguing work is, to the best of my knowledge, the largest PSP single-cell sequencing study to date and only the second overall (Sharma et al., 2021, was the first). I am fascinated by the finding that the REST complex is downregulated, because in our own recently published in vitro studies using human stem-cell derived astrocytes (Fiock et al., 2023), we found that several other regulators of astrocyte identity were downregulated in response to tau uptake.
The finding that tau is upregulated in PSP astrocytes highlights the need for further investigation, since both we (Fiock et al., 2023), and Dr. Kovac's group (Forrest et al., 2023) did not observe any change between diseases or between tau-positive and -negative astrocytes within PSP by RNAscope. Sharma et al. do not comment on such a change in their single-cell data (hopefully one of the authors will comment on this point).
I look forward to seeing both this work and Sharma et al. published and the corresponding datasets made publicly available as they will be an invaluable resource for the neurodegenerative disease research community.
References:
Fiock KL, Hook JN, Hefti MM. Determinants of astrocytic pathology in stem cell models of primary tauopathies. Acta Neuropathol Commun. 2023 Oct 6;11(1):161. PubMed.
Forrest SL, Lee S, Nassir N, Martinez-Valbuena I, Sackmann V, Li J, Ahmed A, Tartaglia MC, Ittner LM, Lang AE, Uddin M, Kovacs GG. Cell-specific MAPT gene expression is preserved in neuronal and glial tau cytopathologies in progressive supranuclear palsy. Acta Neuropathol. 2023 Sep;146(3):395-414. Epub 2023 Jun 24 PubMed.
Sharma A, Song W-M, Farrell K, Whitney K, Zhang B, Crary JF, Pereira AC. Single-cell atlas of progressive supranuclear palsy reveals a distinct hybrid glial cell population. 2021 Apr 12 10.1101/2021.04.11.439393 (version 1) bioRxiv.
Make a Comment
To make a comment you must login or register.