Transcriptomics Paint Astrocytes as Source of Cognitive Resilience
Quick Links
Single-cell transcriptomics aficionados have raised the bar again. In a study published in Nature on July 24, scientists led by Li-Huei Tsai and Manolis Kellis at Massachusetts Institute of Technology, Cambridge, parsed the transcriptomes of 1.3 million cells hailing from six different regions of the human brain. They came from 48 people, including 26 who died with AD. The regions examined corresponded to those successively inundated by tau tangles as the disease progresses. As one might imagine, findings were manifold.
- Transcriptomics study surveyed six brain regions in people with or without AD.
- Reelin-expressing neurons were less abundant in AD.
- Vulnerable neurons in different brain regions were interconnected.
- In astrocytes, greater polyamine and choline synthesis tracked with cognitive resilience.
A few highlights? Neurons appeared to mount wildly different transcriptional responses to disease based on where they resided in the brain. Neurons that relay signals through reelin, an extracellular matrix protein that binds the ApoE receptor, were sparse in the entorhinal cortex and other brain regions in people with AD, suggesting they are vulnerable to the disease. Glial cells responded more consistently across regions, and the researchers pegged astrocytes as the lone cell type in which gene expression synced strongly with cognitive resilience. Specifically, they cranked up production of polyamines and choline, two neuroprotective molecules. The findings suggest factors secreted by astrocytes might bide neurons more time in the face of mounting AD pathology.
“This study is an important step in advancing the field toward brain-wide analysis of cell-type-specific molecular changes in Alzheimer's disease,” wrote Eitan Kaplan of the Allen Institute for Brain Science in Seattle. “Findings like these across many brain regions will be necessary to interpret and treat AD more precisely, as a cell-type and circuit disorder.”
The new analysis joins a line of single-cell studies published by these scientists, who have previously used brain samples from the Religious Orders Study and Memory and Aging Project (ROS-MAP) cohort to dissect how AD influences gene expression in myriad cell types (Oct 2023 news). Previous studies primarily sampled the prefrontal cortex, which neurofibrillary tangles invade only later in the disease. They therefore missed gene expression changes that occur earlier in other regions, such as the entorhinal cortex. What’s more, cells in one region of the brain may respond differently to a given pathological challenge than those in a different cerebral ZIP code.
To tackle regional diversity, co-first authors Hansruedi Mathys, Carles Boix, Leyla Akay, and colleagues isolated nuclei from six regions. The entorhinal cortex is hit by tangles first—in Braak stages I-II—followed by the hippocampus and anterior thalamus in Braak III-IV, then the angular gyrus, midtemporal cortex, and prefrontal cortex in stage V-VI. Based on transcriptomes, the 1.3 million nuclei isolated represented 76 cell types, including 32 excitatory and 23 inhibitory flavors of neuron. Before looking at disease-related responses, the researchers charted the diversity of transcriptional subtypes of neurons and glia in all six brain regions.

Kaleidoscope of Cells. Transcriptomes of 1.3 million nuclei across 14 major cell types revealed 76 subtypes. [Courtesy of Mathys et al., Nature, 2024.]
This atlas in hand, they next asked how AD changes it. First, they looked for vulnerable neurons, namely, those that are scarce in samples from people with AD. Six subtypes of excitatory neuron fit the bill: four in the entorhinal cortex, one in the hippocampus, and one in neocortical regions—at least among people who had tangles there. Two of the entorhinal subtypes expressed high levels of reelin, while all six expressed other components of the reelin signaling pathway. Also up were genes involved in heparin sulfate proteoglycan biosynthesis, a cell surface protein suspected of spreading toxic forms of tau (Holmes et al., 2013).
The scientists found that some of these ill-fated neurons in different brain regions appeared interconnected. For example, vulnerable pyramidal neurons in the hippocampus had synaptic ties to struggling subtypes in the entorhinal cortex and subiculum. The scientists also identified loss of inhibitory neuron subtypes in AD samples, and those also expressed high levels of reelin. The findings jibe with reports of missing reelin-expressing neurons in the AD brain from nearly two decades ago (Aug 2005 news).
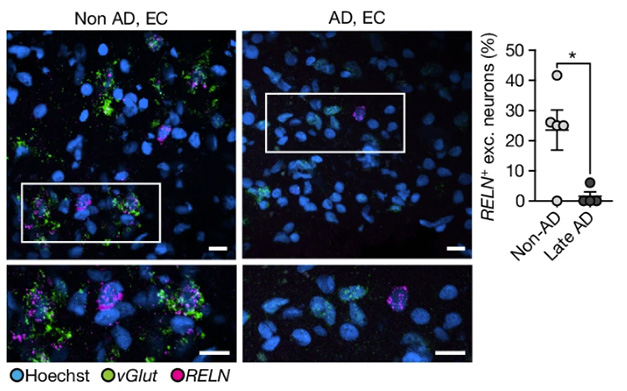
Reelin in the Reaper? In the entorhinal cortex, excitatory neurons expressing reelin (pink) were scarce in people with AD (right) relative to controls (left). Differences were greatest among those with late AD (chart, right). [Courtesy of Mathys et al., Nature, 2024.]
If, or how, reelin signaling determines the fate of these different neuronal subtypes is unclear, Kellis said. Recent studies suggest that reelin signaling is a good thing. A function-boosting variant in the gene may even ward off dementia in carriers of an autosomal-dominant mutation in presenilin-1 (May 2023 news). How reelin’s apparent neuroprotective role relates to the loss of neurons expressing it remains to be investigated, Tsai told Alzforum. Possibly, loss of reelin-expressing cells happens early on, and may itself contribute to AD progression, she noted. Previous studies in mouse models suggest that loss of reelin renders synapses extremely sensitive to even low levels of Aβ aggregates (Jul 2015 news).
How region-specific was the transcriptional response to AD? For neurons, markedly so. Glia, on the other hand, had a more consistent disease-related transcription across regions. Disease signatures in astrocytes overlapped considerably across all the sampled regions, as did those of oligodendrocytes.
Microglia mounted largely overlapping responses within subcortical regions. However, these cells expressed both broad and region-specific signatures. For example, microglia in all regions ramped up clathrin-based endocytosis and turned down viral response genes, but they turned up expression of MHC II only in the entorhinal cortex and hippocampus, and boosted glycolysis only in the entorhinal and prefrontal cortices.
Several AD risk genes identified in GWAS were turned up or down in a region-specific manner in microglia and other cell types. For example, the lipid transporter ABCA7 was enriched in the thalamus in AD; complement receptor CR1, in the neocortex.
A proportion of ROS-MAP participants died while cognitively healthy, yet autopsy revealed ample Aβ plaques and tau tangles. What spared their memories? To address this, the researchers turned to the larger ROS-MAP cohort, which includes snRNA-Seq data from a single region, the prefrontal cortex, from 427 brains. Strikingly, a sub-type of astrocyte was the only cell group that tracked with cognitive resilience. They expressed a cadre of genes, including HMGN2, NQO1, GPX3, and ODC1, which have been tied to antioxidant activity.
The latter encodes the rate-limiting enzyme in the synthesis of polyamines. These nitrogen-packed molecules—notably spermidine—promote autophagy, and have been tied to improved cognition (May 2021 news; May 2021 news).
Daniel Lee, University of Kentucky, Lexington, who studies ties between polyamines and tau deposition, called the new data intriguing. He noted that the so-called polyamine stress response can become activated by tau pathology and a range of other insults, and is a highly regulated, complex pathway. “It may seem that, under certain conditions, astrocytic activation of the PSR is beneficial under the chronic burden of global AD pathology,” he wrote. “However, it would be interesting to learn how the protein expression, polyamines themselves, and their end-product metabolites play out in astrocytes, or on the whole,” he added. He thinks a deeper understanding of the downstream biology of this pathway could point the way to therapeutics.
Choline biosynthesis emerged as another resilience-associated pathway in astrocytes. Genes needed for its synthesis were elevated, while those involved in its breakdown were turned down. Choline helps build the neurotransmitter acetylcholine, which wanes in AD. As phosphatidylcholine, it forms a major component of the lipid membrane. Tsai and Kellis recently reported that choline supplementation corrects lipid imbalances wrought by an AD risk variant in the ABCA7 gene (von Maydell et al., 2024). Choline and polyamine synthesis pathways are intertwined.
Whether these pathways are cause or consequence of cognitive resilience is unclear. Tsai noted that the findings fit with the idea that by churning out neuroprotective compounds, astrocytes might delay cognitive decline wrought by encroaching AD pathology.
Kellis and Tsai view the findings as fuel for hypotheses and further investigation, and said their data will be available for exploration on the interactive website. “We’ve just scratched the surface of these datasets,” Kellis said. “We want others to use them to make more discoveries.”—Jessica Shugart
References
News Citations
- Stunning Detail: Single-Cell Studies Chart Genomic Architecture of AD
- Brain Reelin from Missing Cells in Early AD?
- Reelin Variant Wards Off Dementia in Colombian Kindred Siblings
- Reelin in Aβ Damage?
- Better Living Through Polyamines?
- Polyamines–What Role in Neurodegeneration?
Paper Citations
- Holmes BB, DeVos SL, Kfoury N, Li M, Jacks R, Yanamandra K, Ouidja MO, Brodsky FM, Marasa J, Bagchi DP, Kotzbauer PT, Miller TM, Papy-Garcia D, Diamond MI. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):E3138-47. Epub 2013 Jul 29 PubMed.
- von Maydell D, Wright S, Bonner JM, Staab C, Spitaleri A, Liu L, Pao PC, Yu CJ, Scannail AN, Li M, Boix CA, Mathys H, Leclerc G, Menchaca GS, Welch G, Graziosi A, Leary N, Samaan G, Kellis M, Tsai LH. Single-cell atlas of ABCA7 loss-of-function reveals impaired neuronal respiration via choline-dependent lipid imbalances. bioRxiv. 2024 Jun 28; PubMed.
External Citations
Further Reading
Primary Papers
- Mathys H, Boix CA, Akay LA, Xia Z, Davila-Velderrain J, Ng AP, Jiang X, Abdelhady G, Galani K, Mantero J, Band N, James BT, Babu S, Galiana-Melendez F, Louderback K, Prokopenko D, Tanzi RE, Bennett DA, Tsai LH, Kellis M. Single-cell multiregion dissection of Alzheimer’s disease. Nature, July 24, 2024 Nature
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.