Repeat RNAs Hitchhike to the Ends of Neurons, Attacking Neurites
Quick Links
Scientists know that some RNAs carrying repeat expansions cause problems in cells without even getting translated into protein. The latest part of the neuron discovered to be at risk from repeats is its dendritic arbor, according to scientists in the laboratory of Nancy Bonini at the University of Pennsylvania in Philadelphia. Both the CAG trinucleotide repeats in the huntingtin gene and the ALS- and FTD-linked GGGGCC hexanucleotide repeats of the C9ORF72 gene manage to hitch a ride on transport granules to get into neurites. There, Bonini and colleagues suggest, the RNAs interfere with local translation.
“This work expands the realm of where we see toxic effects due to these hexameric repeat granules,” said Ben Wolozin of Boston University, who was not involved in the paper published December 9, 2015 in eLife.
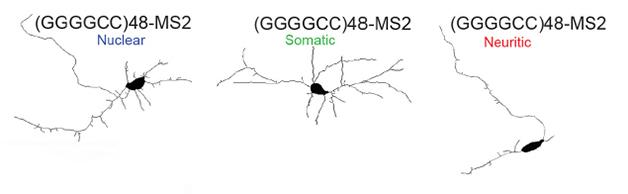
Dendrites defeated. When restricted to the nucleus (left) or soma (center), hexanucleotide repeats leave dendrites unscathed. Shuttled into neurites (right), the repeats prune dendritic arbors. [Courtesy of Burguete et al.]
Both CAG and GGGGCC repeats are toxic to cells, though the exact mechanism is still under investigation (see Jan 2013 conference news; Li et al., 2008). In Bonini’s lab, Alondra Burguete wondered if the RNAs’ effects might be related to their secondary structure. C9ORF72 G4C2 repeats fold up into columns called G-quadruplexes (see Nov 2013 news), while CAG repeats double over into hairpins (de Mezer et al., 2011). Such secondary structures often target RNAs to specific cellular locations for translation (Hamilton and Davis, 2007).
C9ORF72 and CAG repeat RNAs form foci in nuclei (see Jan 2013 conference news), but when Burguete looked more closely, she found them in neurites, too. Using in situ hybridization to study neurons derived from the induced pluripotent stem cells of two C9ORF72 repeat carriers, she detected GGGCC-positive particles in the neurites of three-quarters of the cells. In non-neuronal cells, the repeats stuck to the nucleus or cell body. Similarly, when she expressed 100-CAG repeat RNA expansions in cultured rat spinal cord neurons, the RNAs localized to neuritic granules in about 90 percent of cells. For comparison, she expressed two other rigidly structured RNA repeats in rat neurons—the CUG and CCUG expansions associated with myotonic dystrophy. They localized to neuritic granules as well. In contrast, when she expressed the GAA repeats associated with Friedreich’s ataxia, which form no strong secondary structures, they did not form particles in neurites.
Burguete suspected the particles that repeat RNAS associate with might be transport granules, which shepherd mRNAs along neurites. Indeed, the repeat RNAs co-localized with fragile X mental retardation protein (FMRP), a known component of transport granules. Further supporting this notion, she used live-cell imaging to detect GGGGCC- and CAG-positive granules traveling up and down neurites.
Messenger RNAs bound for dendrites form secondary structures, such as hairpin loops, that scientists believe are important for binding to transport granules. The G-quadruplexes and hairpins assumed by the extended repeats might allow them to sneak aboard the same transport, Burguete theorized.
Those stowaway repeat RNAs caused trouble in the neurites of the rat neurons, Burguete found. Neurons expressing GGGGCC repeats had about four primary neurite branches, compared to the normal six—but only if the repeats made their way to the neurites. Neurons with repeat RNAs in the nucleus or cell body had normal dendritic branching (see image above). To explore this phenomenon in vivo, Burguete studied the epidermal sensory neurons of Drosophila larvae, which produce lush arbors. As with the cultured cells, Burguete observed fewer dendritic arbors in the neurons of larvae expressing 48 GGGGCC repeats.
How did the repeats in transport granules change dendrite branching? The authors suspected that the repeats might interfere with the function of FMRP, which acts locally in neurites to regulate translation of thousands of mRNAs. They examined expression of three FMRP targets: the postsynaptic density protein PSD-95, the cytoplasmic polyadenylation element binding protein CPEB3, and FMRP itself. Sure enough, all were upregulated in induced neurons from C9ORF72 carriers. Other FMRP targets might also be up- or downregulated in the presence of repeat RNAs, Burguete said. Dysregulation of proteins such as PSD-95 could alter the synapse and perhaps explain the dendrite deformities, the authors speculate. If so, then would targeting FMRP fix the problems? In the CCCCGG-expressing fruit flies, downregulating FMRP restored normal branching patterns.
Repeat RNAs might have other effects on RNA traffic or translation in neurites. They might get translated into toxic peptide products in the neurites, Burguete speculated, though she found no C9ORF72 repeat dipeptides or polyglutamine products in her cultured cells.
Wolozin commented that while C9ORF72 hexameric repeats have been associated with RNA granules before, this study newly implicates trinucleotide repeats in this behavior. He was intrigued to see that downregulating FMRP was beneficial, suggesting that drugs could act on FMRP to protect dendrites.
Wolozin and others who spoke with Alzforum noted that the branching defects join a host of problems caused by repetitive RNAs. C9ORF72 repeats encode toxic peptides that may alter the biology of the nucleolus, and either the RNAs or peptides or both interfere with transport between the nucleus and cytosol (see Aug 2015 news; Dec 2014 news; Aug 2014 news). “We have to continue to gather these different mechanisms and try to sort through them,” said Robert Baloh of Cedars-Sinai Medical Center in Los Angeles, who did not participate in the study. “None of them, alone, has stood out as obviously causing the diseases, and it may well be all of them.”
However, some mechanisms seem to be converging. The proteins TDP-43 and FUS, both linked to ALS-FTD, are also involved in RNA processing and transport. TDP-43, for example, helps RNA granules travel along axons (see Feb 2014 news). Wolozin found that TDP-43 mutations cause RNA granules in dendrites to swell and slow down (Liu-Yesucevitz et al., 2014). Dysfunction of the transport granules may turn out to be a key commonality between different mutations that cause ALS-FTD, Burguete speculated.—Amber Dance
References
News Citations
- Chicago—RNA Inclusions Offer Therapeutic Target in ALS
- Researchers Revel in C9ORF72 Advances at RNA Symposium
- ALS Gene Repeats Obstruct Traffic Between Nucleus and Cytoplasm
- Live-Cell Studies Blame Arginine Peptides for C9ORF72’s Crimes
- C9ORF72 Killer Dipeptides Clog the Nucleolus
- Escort Service: A Cytoplasmic Role for TDP-43
Paper Citations
- Li LB, Yu Z, Teng X, Bonini NM. RNA toxicity is a component of ataxin-3 degeneration in Drosophila. Nature. 2008 Jun 19;453(7198):1107-11. Epub 2008 Apr 30 PubMed.
- de Mezer M, Wojciechowska M, Napierala M, Sobczak K, Krzyzosiak WJ. Mutant CAG repeats of Huntingtin transcript fold into hairpins, form nuclear foci and are targets for RNA interference. Nucleic Acids Res. 2011 May;39(9):3852-63. Epub 2011 Jan 18 PubMed.
- Hamilton RS, Davis I. RNA localization signals: deciphering the message with bioinformatics. Semin Cell Dev Biol. 2007 Apr;18(2):178-85. Epub 2007 Feb 12 PubMed.
- Liu-Yesucevitz L, Lin AY, Ebata A, Boon JY, Reid W, Xu YF, Kobrin K, Murphy GJ, Petrucelli L, Wolozin B. ALS-linked mutations enlarge TDP-43-enriched neuronal RNA granules in the dendritic arbor. J Neurosci. 2014 Mar 19;34(12):4167-74. PubMed.
Further Reading
Papers
- Daigle JG, Krishnamurthy K, Ramesh N, Casci I, Monaghan J, McAvoy K, Godfrey EW, Daniel DC, Johnson EM, Monahan Z, Shewmaker F, Pasinelli P, Pandey UB. Pur-alpha regulates cytoplasmic stress granule dynamics and ameliorates FUS toxicity. Acta Neuropathol. 2016 Apr;131(4):605-20. Epub 2016 Jan 4 PubMed.
- Rossi S, Serrano A, Gerbino V, Giorgi A, Di Francesco L, Nencini M, Bozzo F, Schininà ME, Bagni C, Cestra G, Carrì MT, Achsel T, Cozzolino M. Nuclear accumulation of mRNAs underlies G4C2-repeat-induced translational repression in a cellular model of C9orf72 ALS. J Cell Sci. 2015 May 1;128(9):1787-99. Epub 2015 Mar 18 PubMed.
- Cooper-Knock J, Walsh MJ, Higginbottom A, Robin Highley J, Dickman MJ, Edbauer D, Ince PG, Wharton SB, Wilson SA, Kirby J, Hautbergue GM, Shaw PJ. Sequestration of multiple RNA recognition motif-containing proteins by C9orf72 repeat expansions. Brain. 2014 Jul;137(Pt 7):2040-51. Epub 2014 May 27 PubMed.
- Thomas M, Alegre-Abarrategui J, Wade-Martins R. RNA dysfunction and aggrephagy at the centre of an amyotrophic lateral sclerosis/frontotemporal dementia disease continuum. Brain. 2013 May;136(Pt 5):1345-60. PubMed.
- Wojciechowska M, Krzyzosiak WJ. Cellular toxicity of expanded RNA repeats: focus on RNA foci. Hum Mol Genet. 2011 Oct 1;20(19):3811-21. Epub 2011 Jul 4 PubMed.
Primary Papers
- Schweizer Burguete A, Almeida S, Gao FB, Kalb R, Akins MR, Bonini NM. GGGGCC microsatellite RNA is neuritically localized, induces branching defects, and perturbs transport granule function. Elife. 2015 Dec 9;4:e08881. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.