Reactive Astrocytes Boot Basic, Dysfunctional Lysosomes
Quick Links
When astrocytes transform from homeostatic to reactive, what goes wrong inside them? Martin Kampmann, University of California, San Francisco, and colleagues blame dysfunctional lysosomes. In a bioRxiv preprint uploaded on September 12, the scientists reported increased mTOR signaling in reactive astrocyte cultures. Inside the cells’ lysosomes, the pH rose and normal trash disposal stopped. The astrocytes promptly dumped their lysosomes’ contents outside. This lysosomal soup damaged cultured neurons, though how so remains unknown. The work raises the question whether turning down mTOR signaling might be a way to mitigate astrocyte reactivity.
- mTOR signaling increased in cultured reactive astrocytes.
- These cells’ lysosomes become basic, unable to degrade proteins.
- Astrocytes exocytose these lysosomes, whose contents somehow damage cultured neurons.
"The findings are highly relevant because reactive astrocytes are abundant in various [...] diseases including Alzheimer’s, Huntington’s and Parkinson’s, amyotrophic lateral sclerosis and multiple sclerosis," wrote Sagar Gaikwad at University of Texas Medical Branch in Galveston (full comment below).
"This interesting article further explores the role of astrocytes in neurodegeneration, identifying a potential new target for therapeutic intervention," agreed Valentina Fossati of the New York Stem Cell Foundation (full comment below).
In previous work, co-first authors Brendan Rooney and Kun Leng sequenced RNA from human iPSC-derived astrocytes made reactive by a cytokine cocktail. They noticed flawed lysosomes, particularly a dearth of transcripts for vacuolar ATPase proteins that regulate pH and for the hydrolases that chew up cellular waste proteins (Jan 2017 news; Leng et al., 2021).
In the new manuscript, the authors show that these reactive astrocytes have basic, rather than acidic, lysosomes, whose degradative power had waned. The autophagy marker LC3, which lysosomes normally degrade, piled up within the astrocytes.

Limping Lysosomes. In reactive astrocytes (bottom row), lysosomes (first and second columns) are less acidic (third column) and packed with undegraded cellular debris (fourth column). [Courtesy of Rooney et al., bioRxiv, 2021.]
Proteomic analysis confirmed the presence of fewer pH-regulating enzymes and lysosomal hydrolases but more inflammation-related and more exocytosis proteins. In addition, compared to unprovoked astrocytes, reactive astrocytes had twice as much of the lysosomal marker LAMP1 embedded in their cell surface, a proxy for lysosomal exocytosis.
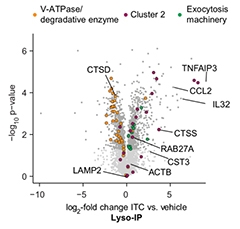
Degradation Down, Exocytosis Up. This volcano plot shows that lysosomes from reactive astrocytes contained fewer vacuolar ATPase and degradative enzymes (orange), but more proteins related to inflammation (purple) and lysosomal exocytosis (green). [Courtesy of Rooney et al., bioRxiv, 2021.]
To find out whether this spewed lysosomal brew affects other cells, Rooney, Leng, and colleagues added medium from these reactive astrocytes to cultured human iPSC-derived neurons. In response, the neurons ramped up caspase 3/7 activity, a sign of apoptosis, and more treated than untreated neurons died, though how the expelled lysosome contents might have been toxic is unclear.
Could this cycle be prevented? Using CRISPR interference, the scientists systematically knocked down some 2,000 genes in reactive astrocytes, then measured lysosome pH with a fluorescent sensor and lysosome exocytosis via cell surface LAMP1. Cells whose knocked-down genes were in the mTOR signaling pathway had more physiological, acidic lysosomes and less lysosomal exocytosis. Ditto for treating cells with an mTOR inhibitor.
Shane Liddelow, New York University, expressed caution. “Given the many ‘neurotoxic compounds’ discovered in culture and never validated in vivo, I am curious to see if these findings can be replicated in models,” he wrote to Alzforum.
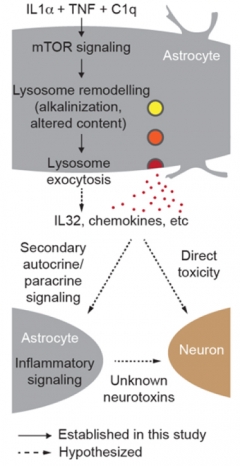
From mTOR to Tox? Astrocytes riled up by cytokines intensify mTOR signaling. This raises lysosome pH and prompts dumping of their contents, creating a neurotoxic environment (solid arrows). It’s unclear if the expelled lysosome soup is toxic directly or indirectly via other pathways (dashed arrows). [Courtesy of Rooney et al., bioRxiv, 2021.]
Carole Escartin, Université Paris-Saclay, CEA, France, is likewise curious whether astrocyte lysosomes and exocytosis change similarly in vivo. “Do they also depend on the mTOR pathway?” she asked (full comment below). The Kampmann group is developing a mouse model with reactive astrocytes to explore mTOR and lysosomal exocytosis inhibitors in neurotoxicity.
Elena Galea, Universitat Autònoma de Barcelona, Spain, questioned whether the lessened neurotoxicity after mTOR inhibition was due to less lysosomal exocytosis. “That can also be interpreted as these drugs rescuing the defective phenotype of ITC-iAstrocytes, which then produce a normal-like supportive astrocyte conditioned medium,” she wrote (full comment below).
For his part, Francisco Quintana, Harvard Medical School, Boston, said the overall findings of the UCSF study align well with his lab’s data. Quintana and colleagues saw a subset of LAMP1-positive astrocytes taken from a mouse model of multiple sclerosis and from people with MS (Sanmarco et al., 2021). In the mice, physical interaction between microglia and astrocytes triggered pathogenic functions, including more mTOR signaling, dysregulated mitochondrial activity, and proinflammatory molecule production (Clark et al., 2021).
“Our and Kampmann’s work converge on similar pathogenic mechanisms within reactive astrocytes through two separate pathways,” Quintana told Alzforum.—Chelsea Weidman Burke
References
News Citations
Paper Citations
- Leng K, Rooney B, Kim H, Xia W, Koontz M, Krawczyk M, Zhang Y, Ullian EM, Fancy SP, Schrag MS, Lippmann ES, Kampmann M. CRISPRi screens in human astrocytes elucidate regulators of distinct inflammatory reactive states. bioRxiv, August 24, 2021
- Sanmarco LM, Wheeler MA, Gutiérrez-Vázquez C, Polonio CM, Linnerbauer M, Pinho-Ribeiro FA, Li Z, Giovannoni F, Batterman KV, Scalisi G, Zandee SE, Heck ES, Alsuwailm M, Rosene DL, Becher B, Chiu IM, Prat A, Quintana FJ. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature. 2021 Feb;590(7846):473-479. Epub 2021 Jan 6 PubMed.
- Clark IC, Gutiérrez-Vázquez C, Wheeler MA, Li Z, Rothhammer V, Linnerbauer M, Sanmarco LM, Guo L, Blain M, Zandee SE, Chao CC, Batterman KV, Schwabenland M, Lotfy P, Tejeda-Velarde A, Hewson P, Manganeli Polonio C, Shultis MW, Salem Y, Tjon EC, Fonseca-Castro PH, Borucki DM, Alves de Lima K, Plasencia A, Abate AR, Rosene DL, Hodgetts KJ, Prinz M, Antel JP, Prat A, Quintana FJ. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science. 2021 Apr 23;372(6540) PubMed.
Further Reading
Papers
- Li Z, Gu Y, Wen R, Shen F, Tian HL, Yang GY, Zhang Z. Lysosome exocytosis is involved in astrocyte ATP release after oxidative stress induced by H2O2. Neurosci Lett. 2019 Jul 13;705:251-258. Epub 2019 Mar 27 PubMed.
- Kim HN, Seo BR, Kim H, Koh JY. Cilostazol restores autophagy flux in bafilomycin A1-treated, cultured cortical astrocytes through lysosomal reacidification: roles of PKA, zinc and metallothionein 3. Sci Rep. 2020 Jun 8;10(1):9175. PubMed.
- Escartin C, Galea E, Lakatos A, O'Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhäuser C, Volterra A, Carmignoto G, Agarwal A, Allen NJ, Araque A, Barbeito L, Barzilai A, Bergles DE, Bonvento G, Butt AM, Chen WT, Cohen-Salmon M, Cunningham C, Deneen B, De Strooper B, Díaz-Castro B, Farina C, Freeman M, Gallo V, Goldman JE, Goldman SA, Götz M, Gutiérrez A, Haydon PG, Heiland DH, Hol EM, Holt MG, Iino M, Kastanenka KV, Kettenmann H, Khakh BS, Koizumi S, Lee CJ, Liddelow SA, MacVicar BA, Magistretti P, Messing A, Mishra A, Molofsky AV, Murai KK, Norris CM, Okada S, Oliet SH, Oliveira JF, Panatier A, Parpura V, Pekna M, Pekny M, Pellerin L, Perea G, Pérez-Nievas BG, Pfrieger FW, Poskanzer KE, Quintana FJ, Ransohoff RM, Riquelme-Perez M, Robel S, Rose CR, Rothstein JD, Rouach N, Rowitch DH, Semyanov A, Sirko S, Sontheimer H, Swanson RA, Vitorica J, Wanner IB, Wood LB, Wu J, Zheng B, Zimmer ER, Zorec R, Sofroniew MV, Verkhratsky A. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021 Mar;24(3):312-325. Epub 2021 Feb 15 PubMed.
Primary Papers
- Rooney B, Leng K, McCarthy F, Rose IV, Herrington KA, Bax S, Chin MY, Fathi S, Leonetti M, Kao AW, Elias JE, Kampmann M. mTOR controls neurotoxic lysosome exocytosis in inflammatory reactive astrocytes. bioRxiv. September 12, 2021
Annotate
To make an annotation you must Login or Register.

Comments
University of Texas Medical Branch
In this important study, using iPSC-derived human astrocytes, Rooney et al. demonstrate that impaired lysosomal acidification is linked to secretion of inflammatory molecules by neurotoxic reactive astrocytes. Additionally, using an elegant pooled CRISPRi screening approach, the authors studied the signaling mechanisms involved in the lysosomal impairments. They found that mTOR is a central upstream regulator of the lysosome alkalinization-exocytosis-neurotoxicity axis in inflammatory reactive astrocytes. These observations are supported by numerous studies that shows mTOR inhibition is beneficial in neurodegenerative disease models and its effects are often attributable to the modulation of autophagy and anti-apoptosis.
The findings are highly relevant because reactive astrocytes are abundant in various human neurodegenerative diseases including Alzheimer’s, Huntington’s and Parkinson’s, amyotrophic lateral sclerosis and multiple sclerosis (Liddelow et al., 2017).
Functions of reactive astrocytes have been a subject of debate, with previous studies showing that they can both hinder and support CNS recovery. Acute astrocyte reactivity in response to injury/stress is beneficial for brain health through axon regeneration (Anderson et al., 2016). Astrocytes also provide trophic support for neurons, promote formation and function of synapses, prune synapses by phagocytosis, and fulfill a range of other homeostatic maintenance functions. On the other hand, sustained or chronic activation of astrocytes promotes neuroinflammation and astrocyte dysfunctions that contribute to neurodegenerative diseases, but less is known about how these astrocytes become dysfunctional.
Indeed, Rooney et al.’s data reveals the patho-mechanism of reactive astrocyte mediated neurotoxicity. The data highlight that inability to maintain lysosomal acidic pH is associated with neurotoxic reactive astrocytes, age-related inflammation—the key events involved in neurodegenerative disease.
However, the results should be concluded with caution because this is just an in vitro study. Future detailed studies are warranted to understand how the lysosomal remodeling in reactive astrocytes influences astrocyte differentiation and functions, neuroinflammation, in context of clinical severity in specific human neurodegenerative diseases. In this context, we and others recently demonstrated that astro-senescence is linked to loss of beneficial function and gain of neurotoxic function, and lysosome impairment contributes to neurodegeneration in Alzheimer’s disease and frontotemporal dementia (Gaikwad et al., 2021; Cohen and Torres, 2019).
Some questions remain to be elucidated. Is the lysosome alkalinization-exocytosis-neurotoxicity axis in inflammatory reactive astrocytes linked to astro-senescence and its dysfunctions and a senescence-activated secretory phenotype? What is the mechanism of lysosome alkalinization in reactive astrocytes during human aging?
In summary, Rooney et al. demonstrated that impaired lysosomal acidification contributes to an inflammatory secretome by neurotoxic reactive astrocytes. The study suggests that a deeper understanding of lysosomal alterations in reactive astrocytes may prove to be critical in understanding neurodegeneration.
References:
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017 Jan 26;541(7638):481-487. Epub 2017 Jan 18 PubMed.
Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016 Apr 14;532(7598):195-200. Epub 2016 Mar 30 PubMed.
Gaikwad S, Puangmalai N, Bittar A, Montalbano M, Garcia S, McAllen S, Bhatt N, Sonawane M, Sengupta U, Kayed R. Tau oligomer induced HMGB1 release contributes to cellular senescence and neuropathology linked to Alzheimer's disease and frontotemporal dementia. Cell Rep. 2021 Jul 20;36(3):109419. PubMed.
Cohen J, Torres C. Astrocyte senescence: Evidence and significance. Aging Cell. 2019 Jun;18(3):e12937. Epub 2019 Feb 27 PubMed.
The New York Stem Cell Foundation
This interesting article further explores the role of astrocytes in neurodegeneration, identifying a potential new target for therapeutic intervention. It is now clear that glial cells play a direct role in damaging and killing neurons in a context of inflammation. We need to expand studies in this direction, because we still know too little about the mechanisms involved.
This work suggests a potential mechanism that seems common to multiple neurodegenerative diseases and is a compelling new target for therapeutic intervention. As this study shows, human models are becoming a crucial addition to in vivo animal models for investigating neurodegenerative diseases. The ability to generate human models of brain cells in a dish is leading to important discoveries, and it is becoming an indispensable addition to the animal studies. We look forward to seeing the evolution of this preprint study.
UMR9199 Neurodegenerative Disease lab
In this bioRxiv paper, Rooney et al. report that astrocytes differentiated from human iPS cells (iAstro) and exposed to a cocktail of cytokines display dysfunctional lysosomes. After one day of treatment, iAstro lysosomes have altered gene expression, are alkalinized and are more prone to exocytosis, releasing yet unknown molecule(s) that directly or indirectly trigger neuronal apoptosis.
The authors used an impressive range of methods including iAstros, several functional assays in vitro, FACS, lysosome-specific proteomics and TIRF microscopy to functionally characterize iAstro lysosomes and their effect on iNeurons. Moreover, they implemented a complex double-readout CRISPR screen to identify genes that can be targeted in reactive astrocytes to correct their defective lysosomes. They identified the mTOR pathway as a positive regulator of this abnormal lysosomal phenotype. The fact that astrocytes perform lysosome-mediated exocytosis was reported previously (Li et al., 2008), but this study describes a new type of functional alteration that astrocytes may acquire when exposed to inflammatory conditions, which has detrimental effects on neurons.
The exploration of human astrocyte defects in diseases using dedicated functional methods is an important future research area identified by a recent consensus paper on reactive astrocytes (Escartin et al., 2021). This study nicely aligns with this central goal for research on reactive astrocytes. However, it is important to consider that the defects described here are triggered in vitro by specific cytokines (as used previously) (Liddelow et al., 2017). It remains to be determined whether such lysosomal dysfunction also occurs in iAstros derived from patients with different genetic and sporadic CNS diseases, but also in situ in patient brains. In the human CNS, does lysosomal alkalinization and exocytosis of toxic compounds represent a widespread dysfunction of reactive astrocytes, or does it occur only in specific disease contexts involving inflammatory cytokine production? Is it observed at early or late stages in progressive diseases? In specific brain regions? Is it also dependent on the mTOR pathway in vivo? These are important questions if this pathway is to be targeted for therapy. Indeed, astrocytes are known to develop disease-specific reactive phenotypes, controlled by specific signaling cascades and genetic networks. For example, a study from our lab recently showed that the JAK2-STAT3 pathway promotes, rather than alters, the proteolytic activity of lysosomes in reactive astrocytes, in the context of Huntington’s disease (Abjean et al., 2021).
This elegant study provides new insight on how excessive inflammatory stimulation of human astrocytes can perturb their cellular functions and trigger neurodegeneration. The Kampmann lab took full advantage of the flexibility and ease of manipulation of human iAstros to deploy a complex, multi-functional assessment of reactive astrocytes. It will be important to determine whether such changes occur in situ, which is not an easy task because most functional assays used to probe lysosomal activity require living samples.
Ultimately, identification of the exact compound(s) mediating neuronal death could open the way to new astrocyte-specific therapies.
References:
Li D, Ropert N, Koulakoff A, Giaume C, Oheim M. Lysosomes are the major vesicular compartment undergoing Ca2+-regulated exocytosis from cortical astrocytes. J Neurosci. 2008 Jul 23;28(30):7648-58. PubMed.
Escartin C, Galea E, Lakatos A, O'Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhäuser C, Volterra A, Carmignoto G, Agarwal A, Allen NJ, Araque A, Barbeito L, Barzilai A, Bergles DE, Bonvento G, Butt AM, Chen WT, Cohen-Salmon M, Cunningham C, Deneen B, De Strooper B, Díaz-Castro B, Farina C, Freeman M, Gallo V, Goldman JE, Goldman SA, Götz M, Gutiérrez A, Haydon PG, Heiland DH, Hol EM, Holt MG, Iino M, Kastanenka KV, Kettenmann H, Khakh BS, Koizumi S, Lee CJ, Liddelow SA, MacVicar BA, Magistretti P, Messing A, Mishra A, Molofsky AV, Murai KK, Norris CM, Okada S, Oliet SH, Oliveira JF, Panatier A, Parpura V, Pekna M, Pekny M, Pellerin L, Perea G, Pérez-Nievas BG, Pfrieger FW, Poskanzer KE, Quintana FJ, Ransohoff RM, Riquelme-Perez M, Robel S, Rose CR, Rothstein JD, Rouach N, Rowitch DH, Semyanov A, Sirko S, Sontheimer H, Swanson RA, Vitorica J, Wanner IB, Wood LB, Wu J, Zheng B, Zimmer ER, Zorec R, Sofroniew MV, Verkhratsky A. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021 Mar;24(3):312-325. Epub 2021 Feb 15 PubMed.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017 Jan 26;541(7638):481-487. Epub 2017 Jan 18 PubMed.
Abjean L, Haim LB, Riquelme-Perez M, Gipchtein P, Derbois C, Palomares MA, Hérard AS, Petit F, Gaillard MC, Guillermier M, Gaudin-Guérif M, Sagar N, Dufour N, Robil N, Kabani M, Melki R, De la Grange P, Bemelmans AP, Bonvento G, Deleuze JF, Hantraye P, Bonnet E, Brohard S, Olaso R, Brouillet E, Carrillo-de Sauvage MA, Escartin C. The JAK2-STAT3 pathway controls a beneficial proteostasis response of reactive astrocytes in Huntington’s disease. bioRxiv. April 29, 2021.
Universitat Autònoma de Barcelona
A main caveat of this study is that it is unclear in which diseases the cellular entity called “inflammatory reactive neurotoxic astrocytes” is relevant. The study is clearly informed by the A1 neurotoxic astrocytes described in a mouse model of septic shock and modelled in vitro with a cocktail of microglia-derived cytokines, here called ITC. The most rigorous interpretation is that ITC-treated iAstrocytes might model astrocytes in septic shock, but caution should be exerted to extend this model and interpretations thereof to any other disease.
Several populations of astrocytes coexist in brain diseases, according to single-nucleus RNA-Seq. In part because there is great overlap among transcriptomes, we still do not know whether these transcriptional states are fate states universal to all brain diseases, although plausibly in different proportions, and, if so, whether they are fundamentally different with regard to function. Thus, the description of ITC-treated iAstrocytes using the rather generic term “inflammatory” is inappropriate. For example, are iAstrocytes inflammatory because they produce cytokines such as IL32? Do these cytokines perform similar roles in the CNS as in the systemic immune system? Is there only one type of “inflammatory” astrocyte in CNS diseases, or do distinct populations release “cytokines” and can be thus described as “inflammatory”?
The term “neurotoxic” borrowed from Liddelow et al. is not appropriate either, because the study by Rooney et al. does not demonstrate that iAstrocytes release a factor or factors neurotoxic for neurons. Upregulation of caspases in neurons treated with iAstrocytes-derived ACM can be explained also by a reduction in the growth-inducing capacity of ITC-treated iAstrocytes, which are ultimately defective cells.
The alteration of lysosomes in ITC-treated iAstrocytes as shown by downregulation of lysosomal-related genes, altered lysosomal content and lysosomal alkalinization, confirms the vulnerability of the endolysosomal system in astrocytes described in models of Alzheimer’s disease (Sollvander et al., 2016; Sanchez-Mico et al., 2021).
I am not convinced yet, however, about the evidence of increased lysosome exocytosis. I leave it to other experts to express their views on whether the uncoupling of dyes documented in Fig. 2a and the accumulation of LAMP1 in the cell surface are unequivocal markers of lysosome exocytosis.
The correlations between reversal of neuronal damage with bafilomycin and mTOR inhibitors could be interpreted to indicate that these drugs rescue the defective phenotype of ITC-iAstrocyte, which then produce a normal-like supportive ACM. I find that the study is over-interpreted following the framework of A1 neurotoxic astrocytes.
Assets of the study include that the CRISPRi screen to uncover pathways involved in lysosomal dysfunction in astrocytes is very elegant. I also find it intriguing that the most dysregulated pathway is OXPHOS, pointing to an interplay between lysosomal and mitochondrial dysfunction in ITC-iAstrocytes.
References:
Söllvander S, Nikitidou E, Brolin R, Söderberg L, Sehlin D, Lannfelt L, Erlandsson A. Accumulation of amyloid-β by astrocytes result in enlarged endosomes and microvesicle-induced apoptosis of neurons. Mol Neurodegener. 2016 May 12;11(1):38. PubMed.
Sanchez-Mico MV, Jimenez S, Gomez-Arboledas A, Muñoz-Castro C, Romero-Molina C, Navarro V, Sanchez-Mejias E, Nuñez-Diaz C, Sanchez-Varo R, Galea E, Davila JC, Vizuete M, Gutierrez A, Vitorica J. Amyloid-β impairs the phagocytosis of dystrophic synapses by astrocytes in Alzheimer's disease. Glia. 2021 Apr;69(4):997-1011. Epub 2020 Dec 7 PubMed.
Make a Comment
To make a comment you must login or register.