New Role for Tau: Making Lipid Droplets in Glia?
Quick Links
Recent studies suggest that when neurons are overwhelmed by oxidative stress, they spew out toxic, peroxidated lipids. Glia then sail in to mop up the spill, containing the oils within intracellular droplets for use later on as fuel. Tau plays an essential role in the production of these droplets, according to a paper in the October Nature Neuroscience. Using a combination of fly and mammalian cell models, scientists led by Hugo Bellen at Baylor College of Medicine in Houston, Texas, found that by virtue of it binding microtubules, tau coaxes newly formed lipid droplets to bud from the endoplasmic reticulum within astrocytes and other glial cells. When tau is either depleted or overexpressed in glia, they fail to form lipid droplets in response to neuronal stress, resulting in an overflow of hazardous lipids. The findings cast deficits in lipid clean-up as an early contributor to AD risk and pathogenesis.
- When stressed neurons spew toxic lipids, glia neutralize them within lipid droplets.
- Glial tau is required. Without it, toxic lipids build up.
- Tau helps droplets bud from the endoplasmic reticulum.
To Jubao Duan of the University of Chicago, the authors provide compelling evidence. That tau dysregulation derails lipid droplets “provides novel mechanistic insight into how accumulation of human tau in the brain may lead to neurodegeneration in AD,” he wrote.
Nancy Bonini of the University of Pennsylvania, Philadelphia, sees broader ramifications. “The role of tau in sequestering of toxic lipids has important implications for the long-term health of the brain, and its susceptibility to disease,” she wrote.
In neurons, oxidative stress occurs when the cells have a glut of reactive oxygen species—essentially free radicals cranked out when the mitochondrial respiratory chain does not burn fuel efficiently. Left unchecked, ROS oxidize fats within neuronal membranes, creating a toxic slurry of peroxidized lipids. Neurons promptly export, and glia import, these toxic molecules, isolating them within lipid droplets. AD risk factors may compromise this cleanup pathway. Previous work from Bellen’s lab and other groups have found that ApoE4 stymies the transfer of peroxidated lipids from neurons into astrocyte lipid droplets (Liu et al., 2017; Qi et al., 2021). Bellen’s group implicated other AD risk genes, as well, including lipid transporters ABCA1, ABCA7, and sorting proteins VPS26 and VPS35 (Moulton et al., 2021). More recently, researchers have separately tied ApoE4 and tau pathology to a glut of lipid droplets in microglia, again suggesting that these little fat droplets might somehow play a central role in AD pathogenesis (Mar 2024 news; Apr 2024 news).
For their new study, first author Lindsey Goodman and colleagues asked how tau might be involved. The scientists deployed a barrage of genetic tinkering in fly and cell culture models. First, they took advantage of the massive compound eye of the fly, in which bundles of retinal photoreceptor neurons are surrounded by doting glial cells, which are similar to astrocytes in mammals. They induced mild ROS in these neurons by conditionally knocking down the mitochondrial protein ND42, which is involved in the electron transport chain. The stressed neurons spurred surrounding glia to crank up production of lipid droplets. When they overexpressed tau in glia, but not in photoreceptor neurons, lipid droplet production fell by nearly 80 percent. When they cranked up tau expression in glia throughout the fly brain, glial lipid droplets plummeted by more than 90 percent, and peroxidated lipids accumulated.
Similarly, in co-cultures of rat primary cells grown in a salt solution that provokes mild oxidative stress, overexpressing tau in astrocytes slashed production of lipid droplets.
Curiously, knocking down glial tau also nipped droplet production in the bud. These flies failed to produce oily deposits when mild neuronal oxidative stress was provoked by knocking down ND42. They climbed more slowly than wild-type flies, slept more during the day, and did not live as long. Restoring normal tau expression in glia corrected these deficits, as did treatment with an antioxidant, suggesting that without glial tau, ROS got out of control and drove the deficits. This rescue failed when glial tau harbored mutations that disrupt its stabilization of microtubules.
To get a closer look at the mechanisms involved, Goodman studied MO3.13 cells—a human oligodendrocyte-like cell line that boasts a massive endoplasmic reticulum. Lipid droplets bud from the ER. To track this, the scientists added fluorescently labeled lipids to MO3.13 cultures, then induced mild oxidative stress. In response, each cell produced about 150 mature lipid droplets, while around 20 immature droplets clung to the surface of the ER (image below). Knocking down tau expression by 70 percent cut the formation of mature droplets by half while doubling the number of immature droplets. This backlog caused the ER to swell, and fluorescent lipids to accumulate within the cells.
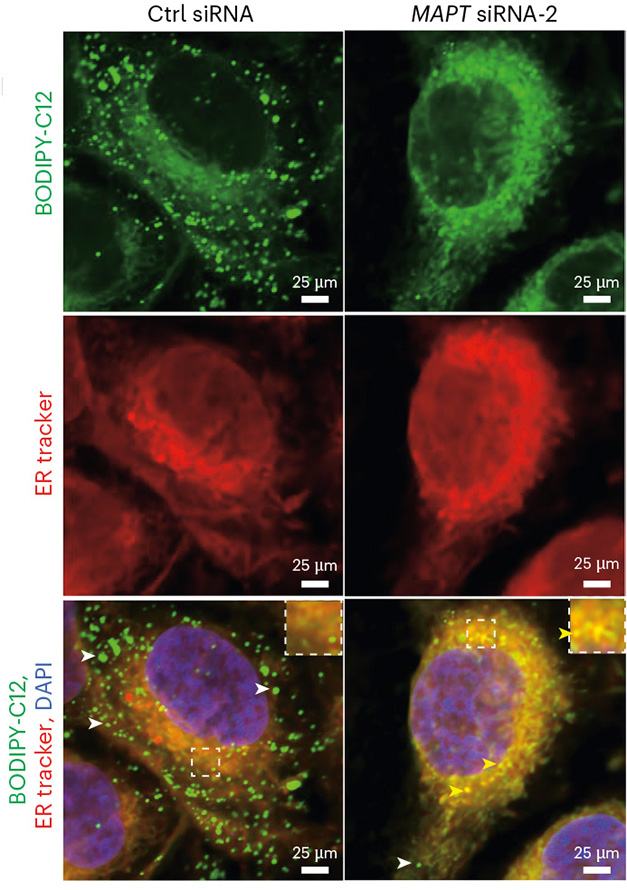
Budding, Interrupted. In response to mild oxidative stress, cultured oligodendrocytes incorporate external lipids (green) into lipid droplets produced by the ER (red). In control cells (left), most of the droplets separate completely from the ER. When tau is knocked down (right), the immature droplets fail to release from the ER (co-localization, yellow). [Courtesy of Goodman et al., Nature Neuroscience, 2024.]
The findings suggest tau’s interaction with microtubules is essential for the efficient budding of lipid droplets from the ER membrane. Indeed, knocking down other microtubule-associated proteins also slowed droplet release from the ER in flies. The role of microtubules may also explain why both too much, and too little, tau doused droplet release. Goodman thinks that having too much overstabilizes microtubules, rendering them too rigid to function. Conversely, without tau, microtubules fall apart. Either way, droplet release falters.
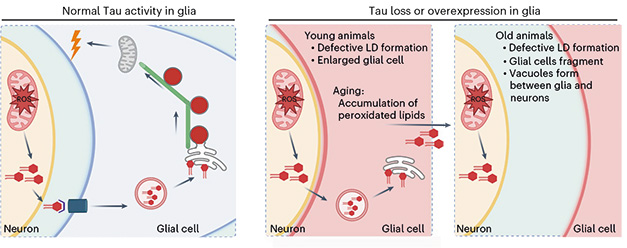
Wrench in the Works? Under healthy conditions (left), stressed neurons hand off peroxidated lipids to glia, which incorporate them into lipid droplets. With the help of tau and microtubules, the droplets bud off the ER membrane and are then consumed as fuel by mitochondria. When tau levels are out of whack (right panels), this pathway derails and lipids accumulate. [Courtesy of Goodman et al., Nature Neuroscience, 2024.]
How might all this play out in AD? Goodman noted that ROS and tau phosphorylation form a vicious cycle, whereby ROS prevents dephosphorylation of tau, and hyperphosphorylated tau promotes ROS (Haque et al., 2019; Alavi Naini et al., 2015). Since hyperphosphorylated tau cannot stabilize microtubules, lipid droplet maturation falters, which renders cells increasingly unable to cope with ROS. Goodman and Bellen place this subpar response to oxidative stress upstream in the AD cascade, when tau phosphorylation begins and ROS start to accumulate. Complicating this scenario, AD risk factors, including ApoE4 and other lipid transporters, might compromise the flow of peroxidated lipid from neurons to glia.—Jessica Shugart
References
News Citations
- Paper Alert: APOE4 Packs on Lipid Droplets in Microglia
- Stirred by Tau, Neurons Amp Up Lipid Droplets in Glia
Paper Citations
- Liu L, MacKenzie KR, Putluri N, Maletić-Savatić M, Bellen HJ. The Glia-Neuron Lactate Shuttle and Elevated ROS Promote Lipid Synthesis in Neurons and Lipid Droplet Accumulation in Glia via APOE/D. Cell Metab. 2017 Nov 7;26(5):719-737.e6. Epub 2017 Sep 28 PubMed.
- Qi G, Mi Y, Shi X, Gu H, Brinton RD, Yin F. ApoE4 Impairs Neuron-Astrocyte Coupling of Fatty Acid Metabolism. Cell Rep. 2021 Jan 5;34(1):108572. PubMed.
- Moulton MJ, Barish S, Ralhan I, Chang J, Goodman LD, Harland JG, Marcogliese PC, Johansson JO, Ioannou MS, Bellen HJ. Neuronal ROS-induced glial lipid droplet formation is altered by loss of Alzheimer's disease-associated genes. Proc Natl Acad Sci U S A. 2021 Dec 28;118(52) PubMed.
- Haque MM, Murale DP, Kim YK, Lee JS. Crosstalk between Oxidative Stress and Tauopathy. Int J Mol Sci. 2019 Apr 22;20(8) PubMed.
- Alavi Naini SM, Soussi-Yanicostas N. Tau Hyperphosphorylation and Oxidative Stress, a Critical Vicious Circle in Neurodegenerative Tauopathies?. Oxid Med Cell Longev. 2015;2015:151979. Epub 2015 Oct 20 PubMed.
Further Reading
Papers
- Mi Y, Qi G, Vitali F, Shang Y, Raikes AC, Wang T, Jin Y, Brinton RD, Gu H, Yin F. Loss of fatty acid degradation by astrocytic mitochondria triggers neuroinflammation and neurodegeneration. Nat Metab. 2023 Mar;5(3):445-465. Epub 2023 Mar 23 PubMed.
Primary Papers
- Goodman LD, Ralhan I, Li X, Lu S, Moulton MJ, Park YJ, Zhao P, Kanca O, Ghaderpour Taleghani ZS, Jacquemyn J, Shulman JM, Ando K, Sun K, Ioannou MS, Bellen HJ. Tau is required for glial lipid droplet formation and resistance to neuronal oxidative stress. Nat Neurosci. 2024 Oct;27(10):1918-1933. Epub 2024 Aug 26 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.