Stirred by Tau, Neurons Amp Up Lipid Droplets in Glia
Quick Links
Lipid droplets accumulate in microglia in mouse and cell models of amyloidosis but scientists are not sure why. Evidence suggests that Aβ helps create the glut, but now scientists led by Lingyan Shi and Xu Chen at the University of California, San Diego, report that mutant tau lends a hand. In the April 23 Cell Metabolism, they show that, in mouse, fly, and cell models of tauopathy, neurons shuttle lipids to microglia, where they form droplets and trigger neuroinflammation. Neuronal AMP-activated protein kinase modulates this process, the authors found. Knocking it out exacerbated lipid droplets in the glia, while activating the kinase alleviated them. However, it’s unclear if this lipid regulation is linked to phosphorylation of tau or of the many other AMPK substrates.
- In models of tauopathy, neurons produce more lipid yet metabolize less.
- Instead, they pass the lipids to microglia.
- AMP kinase attenuates this process.
“[This] work beautifully demonstrates how upregulated lipid synthesis and downregulated lipid turnover leads to pathological lipid accumulation in both neurons and glia in multiple tauopathy model systems,” wrote Priyanka Narayan and Roxan Stephenson, National Institutes of Health in Bethesda, Maryland.
Amita Sehgal at the University of Pennsylvania in Philadelphia was excited by these findings. “[They] bolster the growing body of work that neurons transfer lipids to glia, most likely to alleviate their own oxidative damage,” she wrote. Earlier this year, Sehgal reported that, in wild-type fruit flies, neurons offload oxidized lipids to glia, which clear the fat droplets during sleep (Feb 2024 news).
In the aging mouse hippocampus, the number of lipid droplet-accumulating microglia tick up. These poorly clear debris, while spewing inflammatory cytokines (Aug 2019 news). Both Aβ and APOE4 exacerbate microglial lipid droplet accumulation (Mar 2024 news).
To see what happens in tauopathy models, first author Yajuan Li used Raman spectroscopy to measure lipids within hippocampal tissue of PS19 mice. Raman spectroscopy uses two lasers to jiggle molecular bonds, allowing scientists to measure vibrational energies emitted and chart the distribution of molecules in cells without needing to stain for lipids.
Within the hippocampi of 9-month-old PS19 mice, about three months after neurofibrillary tangles had formed, lipid droplets accumulated, mainly in microglia (image below) and especially in those surrounding synapses containing phospho-tau inclusions. Droplet load tightly correlated with hippocampal neuron loss. Lipid-filled microglia appeared to be under oxidative stress, as indicated by a high FAD-to-NADH ratio. These are among the major redox coenzymes, and measuring both gives a more complete snapshot of mitochondrial burden than measuring either alone (Chance et al., 1979). Being in oxidized and reduced forms, respectively, a high FAD/NADH ratio indicates a more oxidative state.

Fatty Microglia. In PS19 hippocampal tissue, lipid droplets (light blue puncta) mostly accumulated in microglia (purple, first panel), though some occurred in astrocytes (purple, second panel). Oligodendrocytes (purple, third panel) and neurons (purple, fourth panel) had almost none. Arrows indicate lipid droplets within cells. [Courtesy of Li et al., Cell Metabolism, 2024.]
Suspecting the microglial lipids came from neurons, the authors tested if human iPSC-derived neurons carrying the frontotemporal dementia V337M variant of tau could pass lipids to mouse microglia. Within 24 hours of bathing in conditioned media from tauopathy neurons, but not wild-type neurons, microglia accumulated lipid droplets (image below). Their FAD/NADH ratio inched up, they poorly phagocytosed fluorescent beads, and they ramped up expression of inflammatory cytokines—all features of droplet-laden microglia seen in AD models. “This study adds a new source—neurons—as a cause of lipid-droplet accumulating microglia in neurodegeneration,” wrote Michael Haney, University of Pennsylvania (comment below).
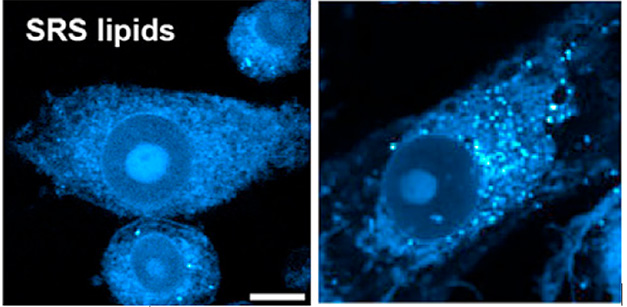
Lipid Media? Unlike microglia grown in control media (left), those grown in media from tauopathy iPSC neurons (right) accumulated lipid droplets (blue puncta). [Courtesy of Li et al., Cell Metabolism, 2024.]
Curiously, the V337M tau neurons made more lipids, and degraded fewer, than did wild-type neurons, as determined by isotope labeling. They also had 10 times more lipid droplets and 1.4-fold higher FAD/NADH ratio. The authors believe that tauopathy perturbs lipid processing, and that in the brain, neurons protect themselves by offloading the excess fats to microglia.
To track that lipid turnover, the scientists turned to a fruit fly model of tauopathy, using deuterium oxide labeling to enhance lipid detection by Raman spectroscopy. D2O introduced in their chow incorporated into lipids, which then cleared when the scientists deprived the flies of food. Microglia in tauopathy flies accumulated larger lipid droplets than glia in wild-type flies yet barely cleared lipids when food was withdrawn, whereas wild-type flies halved their deuterium-lipid load.
This enhanced lipid synthesis and impaired breakdown aligns with altered gene expression patterns that Li and colleagues found when analyzing published RNA-Seq data (Sjöstedt et al., 2020). Compared to control tissue, AD brains upregulated genes involved in lipid synthesis and transfer, such as LPIN1 and ABCA1. Among the most downregulated was PRKAA, which encodes the α subunit of AMP kinase.
This protein caught the researchers’ attention because AMPK is crucial for lipid metabolism. It is also a tau kinase. Notably, in the cortices of 9-month-old PS19 mice, there was less phosphorylated AMPK, the active form, than in control brain. V337M iPSC neurons also had less p-AMPK and less phosphorylated AMPK substrates than did wild-type neurons, suggesting that AMPK is less active in tauopathy brains and neurons.
Did AMPK affect lipid droplet formation? Indeed, giving wild-type iPSC neurons an AMPK inhibitor ramped up expression of lipid synthesis genes, and droplets accumulated. Knocking out AMPK in 5-month-old PS19 tauopathy mice, which do not yet have tangles, did the same.
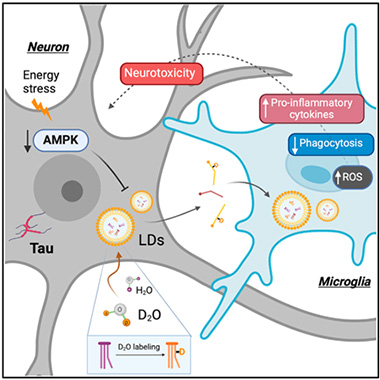
Death by Fat. In tauopathy neurons (gray), downregulated AMP kinase causes lipid droplets (LDs, yellow) to accumulate, forcing the cells to export the fats to microglia (blue). This triggers oxidative stress, suppresses phagocytosis, and ramps up production of neuroinflammatory cytokines. [Courtesy of Li et al., Cell Metabolism, 2024.]
In contrast, giving V337M tau neurons a small molecule that activates AMPK, or overexpressing the kinase in tauopathy flies, downregulated lipogenesis gene expression, accelerated lipid metabolism, and reduced lipid droplet load. Taken together, these results suggest that AMPK prevents lipid synthesis and promotes their clearance.
All told, tauopathy spurs neurons to make more lipid and break down less, inundating them with fat that they offload to microglia as droplets. This, in turn, weakens microglia (image above).
“The findings highlight that targeting pathways that cause lipid accumulation in various cell types in neurodegenerative diseases is an important field for therapeutic development,” wrote Ole Isacson and Penny Hallett of Harvard Medical School, Boston (comment below).
Chen said they are exploring pathways beyond AMPK that regulate lipid droplets to see if molecules downstream, or specific to microglia, might be good drug targets.—Chelsea Weidman Burke
References
News Citations
- While a Fly Sleeps, Its Glia Burn Neuronal Lipids to Refresh the Brain
- Newly Identified Microglia Contain Lipid Droplets, Harm Brain
- Paper Alert: APOE4 Packs on Lipid Droplets in Microglia
Research Models Citations
Mutations Citations
Paper Citations
- Chance B, Schoener B, Oshino R, Itshak F, Nakase Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J Biol Chem. 1979 Jun 10;254(11):4764-71. PubMed.
- Sjöstedt E, Zhong W, Fagerberg L, Karlsson M, Mitsios N, Adori C, Oksvold P, Edfors F, Limiszewska A, Hikmet F, Huang J, Du Y, Lin L, Dong Z, Yang L, Liu X, Jiang H, Xu X, Wang J, Yang H, Bolund L, Mardinoglu A, Zhang C, von Feilitzen K, Lindskog C, Pontén F, Luo Y, Hökfelt T, Uhlén M, Mulder J. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science. 2020 Mar 6;367(6482) PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Li Y, Munoz-Mayorga D, Nie Y, Kang N, Tao Y, Lagerwall J, Pernaci C, Curtin G, Coufal NG, Mertens J, Shi L, Chen X. Microglial lipid droplet accumulation in tauopathy brain is regulated by neuronal AMPK. Cell Metab. 2024 Jun 4;36(6):1351-1370.e8. Epub 2024 Apr 23 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.