Do Lysosomes Help Propagate Tau Seeds?
Quick Links
Aggregated tau can be passed between cells in tiny vesicles known as exosomes. Once these lipid bubbles are inside a new cell, how does tau escape them? In the January 8 Acta Neuropathologica, researchers led by Jürgen Götz at the University of Queensland, Brisbane, Australia, propose that the cell’s degradation machinery opens the door. The authors found that, in cultured cells, internalized exosomes containing aggregated tau ended up inside lysosomes. Alas, rather than digesting this material, lysosomes ruptured and spilled their contents, sparking the aggregation of cytosolic tau. Götz believes this rupture happens because exosomes resist digestion, forcing lysosomes to become more acidic to try to clear them. Eventually, this acidity ruptures the exosomes, but also the lysosomal membrane. In support of this idea, blocking lysosomal activity prevented tau seeding.
- Exosomes laden with tau aggregates are taken up by the endolysosomal system.
- There, they rupture lysosomes, spilling tau seeds into the cytosol.
- Blocking lysosomal activity prevented aggregation of endogenous tau.
“Exosomal tau seeds induce endosomal permeabilization through lysosomes, facilitating access to the cytosol,” Götz wrote to Alzforum. He believes the same could occur with other aggregated proteins, as well.
Ralph Nixon at New York University agreed that it makes sense for extracellular tau to reach the cytosol via the endosomal-lysosomal system. Because exosomes are harder to digest than free aggregated tau, this packaging may promote the spread of pathogenic tau, he noted.
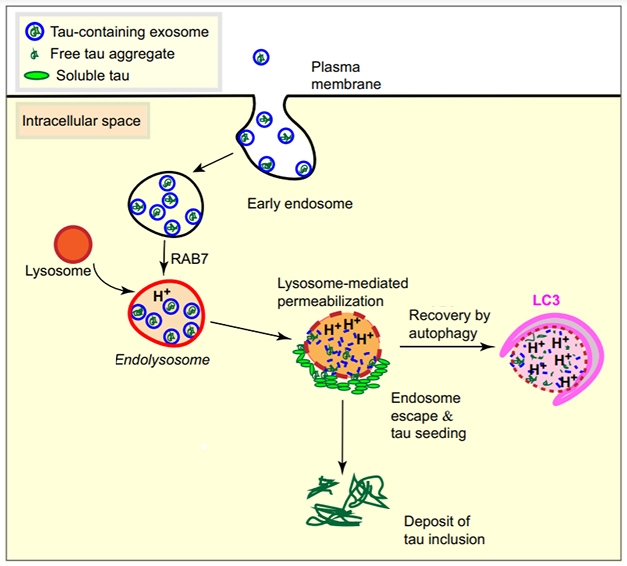
Lysosomal Breakdown. Internalized tau-laden exosomes end up in lysosomes, where they clog up digestion and rupture the membrane. This allows tau to escape into the cytosol to spark new aggregation. [Courtesy of Polanco et al., Acta Neuropathologica.]
Exosomes and larger vesicles have been found to deliver aggregated tau and other proteins to neurons both in vitro and in vivo (Dec 2014 conference news; Dec 2020 news). Götz and colleagues wanted to know how uptake of exosomes facilitates seeding. First author Juan Carlos Polanco added exosomes isolated from the brains of rTg4510 mice to cultured cells that detect tau aggregation via fluorescence resonance energy transfer (FRET) (Oct 2014 news). The exosomes were taken up by endosomes (see model above). After 24 hours, about three-quarters of these laden endosomes had fused with lysosomes, as seen by expression of the marker LAMP1 and a drop in pH. Tau deposits formed around the acidic lysosomes (see image below).

Tau Seeding. Tau-laden exosomes (blue) end up inside lysosomes (pink). In many cells (dotted lines), this triggers new tau aggregation (green, arrowheads). Rightmost panel shows all three. [Courtesy of Polanco et al., Acta Neuropathologica.]
Alkanizing lysosomes put a stop to this. Simple addition of ammonium chloride to the cell culture cut tau aggregation by two-thirds. Similarly, suppressing RAB7, an endosomal membrane protein needed for fusion with lysosomes, dropped aggregation in half, whereas overexpressing RAB7 boosted tau aggregation.
How might lysosomes spur aggregation? Exosomes are highly stable vesicles, containing large amounts of sphingomyelin, cholesterol, and di-saturated lipids that resist breakdown (Zaborowski et al., 2015; Skotland et al., 2017). The authors theorized that these indigestible masses would force lysosomes to crank up their digestive juices so much that they would end up punching holes in their own membranes.

Shattered Shell. In a mouse primary neuron (nucleus blue), galectin (red) binds to the broken innards of an endosome (inset) that once harbored an exosome (green). [Courtesy of Polanco et al., Acta Neuropathologica.]
To test this idea, Polanco and colleagues added fluorescent galectin, a protein that binds to glycoconjugates sequestered inside endosomes. In control cultures, galectin remained diffuse and unbound, unable to reach these glycoconjugates. In cultures fed exosomes, however, galectin bound to the exosomal-laden endolysosomes, indicating that these vesicles had broken open (see image at left). Intriguingly, galectin bound equally well whether the exosomes contained tau aggregates or not. In other words, exosomes alone cause endolysosome disruption, regardless of the exosome’s contents.
Not all endosomes stuffed with exosomes broke open, suggesting some physiological threshold has to be reached. Conversely, though, in every case where a tau-laden exosome sparked aggregation, endolysosomal rupture had occurred. This emphasizes that lysosomes have to be rendered permeable for tau seeds to spread. Importantly, lysosomes also break in mouse primary hippocampal neurons treated with exosomes, hinting that the process could be at work in the brain.
Nixon pointed out, however, that endolysosomal rupture might be due to weak, rather than excessive, acidification. As lysosomes malfunction in Alzheimer’s disease and other disorders, they become less acidic and able to digest substrates (Jun 2010 news; May 2011 news). When these lysosomes encounter a mass of exosomes, digestion simply stalls. Gradually, in the acidic environment of the lysosome, resistant substrates oxidize. This oxidation then damages the lysosome membrane, allowing access to the cytosol, Nixon proposed.
The distinction could be key, since Nixon’s model implies that blocking lysosomal function would do further harm. Instead, promoting lysosomal digestion might be helpful therapeutically. “The bottom line is to keep the lysosome healthy,” Nixon said.—Madolyn Bowman Rogers
References
News Citations
- Exosomes: Purveyors of Neurodegenerative Disease?
- Fatal Gift Wrap: Neurons Fall for Packaged Tau Oligomers
- Cellular Biosensor Detects Tau Seeds Long Before They Sprout Pathology
- Death of the Neatnik: Neurons Perish When Trash Clutters Their Space?
- Lysosomal Block Clogs Transport, Swells Neurites
Research Models Citations
Paper Citations
- Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience. 2015 Aug 1;65(8):783-797. Epub 2015 Jun 26 PubMed.
- Skotland T, Sandvig K, Llorente A. Lipids in exosomes: Current knowledge and the way forward. Prog Lipid Res. 2017 Apr;66:30-41. Epub 2017 Mar 23 PubMed.
Further Reading
Primary Papers
- Polanco JC, Hand GR, Briner A, Li C, Götz J. Exosomes induce endolysosomal permeabilization as a gateway by which exosomal tau seeds escape into the cytosol. Acta Neuropathol. 2021 Feb;141(2):235-256. Epub 2021 Jan 8 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.