Could “Big Tau” Protect Brain Regions from Tangles?
Quick Links
In Alzheimer’s disease, tau tangles accumulate in the temporal lobe and cortex, but spare other regions such as the cerebellum and brainstem. In a July 31 preprint on bioRxiv, scientists led by Huda Zoghbi at Baylor College of Medicine, Houston, offered an explanation. They homed in on a splice variant known as “big tau,” almost twice the size of the other isoforms, that is common in the peripheral nervous system. To their surprise, they found the behemoth in the cerebella and brainstems of wild-type mice. In vitro studies showed that it resisted hyperphosphorylation and aggregation, and was degraded more readily than other forms of tau. To the authors, this implied that big tau might help protect the brain from tangles. In support of this, they found more of it in the cerebella, but not the cortices, of AD patients than of healthy controls.
- A big tau variant is found in tangle-resistant brain regions such as the cerebellum.
- This isoform degrades quickly and resists phosphorylation and aggregation.
- In AD patients, big tau production revs up in the cerebellum, but not the cortex.
Others agreed the data are worth following up. “A better understanding of how this isoform interacts with aggregates, and its role in neuronal physiology, is very much warranted,” William McEwan at the University of Cambridge wrote to Alzforum. Itzhak Fischer at Drexel University College of Medicine, Philadelphia, noted the findings could be used to develop tau therapies. “I was impressed with the quality and importance of this study and with the physiological and clinical implications of the results,” Fischer wrote (comments below).
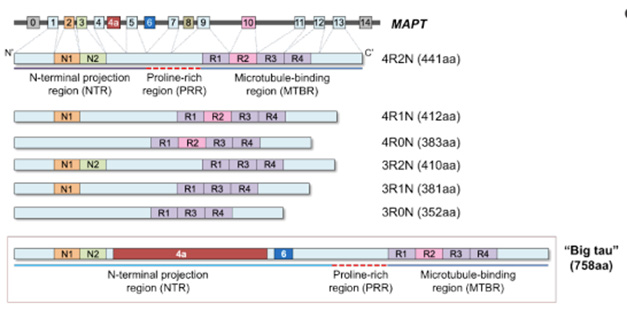
Super-Size Me. Big tau (bottom) contains two additional exons, 4a and 6, in its N-terminal region compared with the next-largest species, 4R2N tau (top). [Courtesy of Chung et al., bioRxiv.]
In human brain, there are six common tau isoforms, as determined by the inclusion of either no, one, or two N-terminal domains, and either three or four microtubule-binding regions. The largest of these species, 4R2N tau, is 441 amino acids long. However, the relatively understudied “big tau” variant tops out at 758 amino acids, due to the inclusion of two additional N-terminal exons (image above). Since its discovery in the 1990s, this super-sized tau has been thought to be mainly a peripheral protein (Goedert et al., 1992; Couchie et al., 1992; Georgieff et al., 1993).
Zoghbi and colleagues did not set out to study big tau, but instead came across it while comparing the distribution of tau isoforms across brain regions. First author Dah-eun Chloe Chung identified the large isoform in the cerebella and brainstems of 6-month-old wild-type mice. Quantitative reverse transcription PCR, using primers specific for the big tau splice junction, showed this transcript made up 10 to 15 percent of tau in the cerebellum and brainstem, but was 10-fold less abundant elsewhere—about 1 percent of cortical tau was big tau. To measure protein levels, the authors generated antibodies specific for both mouse and human versions of the giant protein. The mouse antibody measured about threefold more protein in cerebellum and brainstem than in the cortex.
The authors wondered if the presence of big tau explained the resistance of these regions to tangles. Several observations supported this. For one, this isoform clings more strongly to microtubules. The authors identified five new microtubule-binding motifs in its unique segments, and found it was able to displace 4R2N tau in competitive binding assays. Big tau also degrades more easily. It contains 20 ubiquitination sites absent in shorter forms, accumulates more ubiquitin tags, and is eliminated from cells twice as fast. Perhaps for these reasons, in aging mice the supersized tau did not become hyperphosphorylated.
All these factors should result in less aggregation. To test this, the authors expressed 4R2N or big tau, each containing the aggregation-prone P301L mutation, in a cellular seeding assay. 4R2N formed three times as much aggregate as did the larger tau. Likewise, when the authors expressed both species in the brains of newborn mice using an adenoviral vector, 4R2N tau caused 15 times more insoluble deposits six months later.
To examine the relevance to people, the authors compared tau transcripts in the cerebella and middle temporal gyri of 11 AD patients and eight healthy controls. In control brain, the cerebellum expressed about twice as much big tau as did the temporal cortex, but in AD brain, the difference swelled to sixfold, suggesting production of the large isoform amped up. At the protein level, there was about twice as much of the large variant in the cerebella of AD patients as in control cerebella.
Fischer noted that previous work of his and others had predicted that big tau might be protective against tau misfolding (e.g., Fischer and Baas, 2020). “What was missing was an in vivo confirmation and data from experimental models of degeneration, as well as supportive data from human tissue. These new findings provide that important new information,” Fischer wrote.
For their part, the authors suggested using anti-sense oligonucleotides against specific tau splice variants to enhance the relative production of the large protein. They plan to explore whether that ameliorates tau pathology in mouse models. In addition, Zoghbi is collaborating with Alison Goate at Icahn School of Medicine, Mount Sinai, New York to investigate whether people who are resilient to AD pathology have more big tau in their brains.—Madolyn Bowman Rogers
References
Mutations Citations
Paper Citations
- Goedert M, Spillantini MG, Crowther RA. Cloning of a big tau microtubule-associated protein characteristic of the peripheral nervous system. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1983-7. PubMed.
- Couchie D, Mavilia C, Georgieff IS, Liem RK, Shelanski ML, Nunez J. Primary structure of high molecular weight tau present in the peripheral nervous system. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4378-81. PubMed.
- Georgieff IS, Liem RK, Couchie D, Mavilia C, Nunez J, Shelanski ML. Expression of high molecular weight tau in the central and peripheral nervous systems. J Cell Sci. 1993 Jul;105 ( Pt 3):729-37. PubMed.
- Fischer I, Baas PW. Resurrecting the Mysteries of Big Tau. Trends Neurosci. 2020 Jul;43(7):493-504. Epub 2020 May 17 PubMed.
Further Reading
Primary Papers
- Chung D-e, Deng X, Yalamanchili HK, Revelli J-P, Han AL, Tadros B, Richman R, Dias M, AlaviNaini F, Boeynaems S, Hyman BT, Zoghbi HY. The big tau splice isoform resists Alzheimer's-related pathological changes. 2024 Jul 31 10.1101/2024.07.30.605685 (version 1) bioRxiv.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.