Building Better Mouse Models for Late-Onset Alzheimer’s
Quick Links
Why do so many promising Alzheimer’s therapies perform well in mice but fail in clinical trials? Scientists lay some of the blame on the lack of good mouse models for late-onset disease, as well as discrepancies between the outcomes used in animal research versus those used in trials. Now, the National Institute on Aging has funded a five-year, $25 million research center to try to remedy this situation. Scientists at Indiana University, The Jackson Laboratory, and Sage Bionetworks will collaborate to generate and characterize numerous mouse models that express genetic variants linked to late-onset AD, seeking those that most faithfully represent various aspects of the human disease. The researchers will advance the most promising models into a preclinical testing pipeline, along the way developing best practices that mimic the design of human trials.
The ultimate goal is to speed up development of Alzheimer’s therapeutics, said Bruce Lamb at Indiana University, Indianapolis, who leads the center, dubbed Model Organism Development and Evaluation for Late-onset AD (MODEL-AD). Lamb encourages researchers to submit ideas for models to make and drugs to test. “We hope to engage the research community,” he told Alzforum. All data and models generated will be made freely available to researchers in academia, industry, and other institutions, he added. Researchers will be able to submit ideas through the MODEL-AD website. Symposia on the project are being planned for upcoming conferences.
Researchers applauded the project, noting that the field needs Alzheimer’s models that go beyond amyloid and tau pathology. “Having different types of models to address a greater diversity of questions will be of great benefit,” Thomas Wisniewski at New York University Langone Medical Center told Alzforum. Guojun Bu at the Mayo Clinic in Jacksonville, Florida, agreed, “We need innovative model systems that better represent late-onset Alzheimer’s. I’m excited about this.”
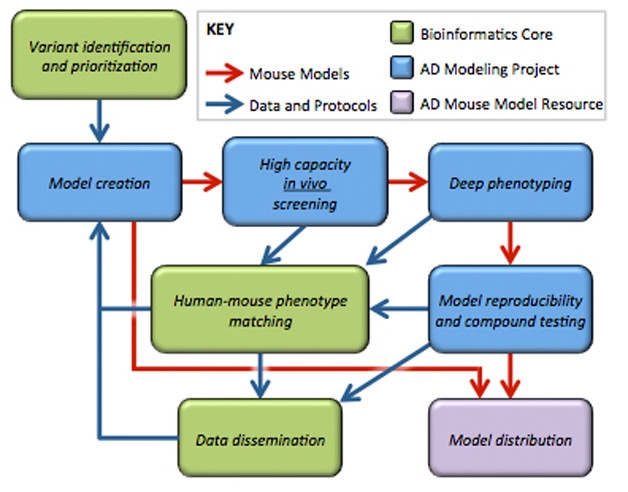
Iterative Process. Phenotypic data from initial models will inform the generation of better ones. The most promising models will advance to preclinical testing and widespread distribution. [Courtesy of Michael Sasner.]
Beyond Amyloid and Tau
Most current mouse models express mutant versions of human APP or tau, which in people lead to early onset AD or frontotemporal dementia, respectively. Researchers agree these models have been instrumental for understanding amyloid and tau pathology, and will continue to be useful for research. However, they do not represent the full spectrum of the human disease. In particular, the underlying molecular mechanisms in these animals may not resemble those at work in late-onset AD, which makes up 95 percent or more of cases and has various genetic and environmental causes.
Researchers have long known about the shortcomings of current models. The idea for the new center first arose at a 2010 NIA workshop on improving preclinical therapeutic development, noted Lorenzo Refolo, who directs the AD drug discovery program at the NIA in Bethesda, Maryland. Scientists participating in the 2012 and 2015 National Institutes of Health AD research summits fleshed out recommendations (see Feb 2015 news series), which will be put into practice in the new center, Refolo said.
The time is ripe for this project. Advances in next-generation sequencing and genome editing make it feasible, Lamb noted. The former is turning up many new AD risk factors as well as molecular pathways involved in the disease. This information can be incorporated into models. The latter, including CRISPR, make it possible to rapidly and cheaply generate specific genetic mutations in mice (see Sep 2014 news series).
The MODEL-AD center includes three facilities. A bioinformatics core, directed by Greg Carter at The Jackson Laboratory, Bar Harbor, Maine, and Andrew Saykin at Indiana, will analyze big-data projects such as ADNI, AMP-AD, and M2OVE-AD, and propose genetic variants for inclusion in models (see Feb 2014 community news). Gareth Howell and Michael Sasner at JAX lead the disease modeling core, which will generate the mice and phenotype them with scientists at Indiana. Finally, a preclinical core led by Paul Territo at Indiana and Stacey Rizzo at JAX will develop the testing pipeline. Sage Bionetworks, a nonprofit research organization in Seattle, will make the data available through the NIA-supported AMP-AD Knowledge Portal.
Multitude of Models
The MODEL-AD center aims to develop eight models per year for the next five years, screening each for Alzheimer’s pathology and advancing the most promising ones for thorough phenotyping. Genetic variants will be knocked in, resulting in physiological expression levels that may model human disease better than overexpression models do (see Dec 2016 conference news). The first model to be created will combine the two strongest genetic risk factors for late-onset disease, ApoE4 and the R47H variant of TREM2, Howell told Alzforum. If the animals do not develop a strong Alzheimer’s phenotype, the researchers will add additional risk factors, such as variants in the microglial transporter ABCA7 and the immune gene interleukin 1 receptor accessory protein (IL1RAP). Both these proteins help clear amyloid, with mutations boosting amyloid buildup (see Mar 2015 news; Ramanan et al., 2015). Howell said the researchers would consider humanizing the APP and tau genes if needed, or even using mutant versions of these genes in some models to achieve greater pathology. “It’s difficult to know the right combinations of alleles. We don’t know what it takes to develop late-onset AD,” Howell noted. Data from the first models will inform the generation of later lines in an iterative process that researchers hope will bring them closer and closer to the human disease (see image above).
Researchers acknowledged that it may be challenging to generate a model of full-blown Alzheimer’s using risk genes in such a short-lived species as mice. Even so, the models may be useful even if they do not develop florid plaques and tangles, said Eliezer Masliah, who heads NIA’s Division of Neuroscience. The mice may still reproduce molecular signatures characteristic of late-onset AD, providing clues to the basic biology of the disease and to potential therapeutic targets. The researchers will analyze gene expression, proteomics, and epigenetic signatures in promising models, comparing them to human data to look for biomarker similarities and discover what aspect of disease each mouse mimics best.
Commenters called this a strength of the approach. “There are definitely Aβ-independent pathways that we could tease out in these mouse models in the absence of amyloid,” Bu suggested. Philip De Jager at Brigham and Women’s Hospital, Boston, noted that because no single mouse model is likely to recapitulate the whole disease, researchers need a range of models that encompass multiple different mechanisms. In particular, the field could use AD models driven by inflammatory changes, De Jager added. Wisniewski agreed that late-onset AD probably has diverse causes, and predicted there are subtypes that may look quite different at a molecular level and require a distinct therapeutic approach, which could be tested in an appropriate mouse.
The researchers will also characterize mice with brain imaging and fluid markers to derive biomarker data comparable to that used in human studies. The hope is that these biomarkers could be used to stage the mice in accordance with human disease (see Feb 2013 conference news; Aug 2016 conference news). In the first year, the center will phenotype and stage some existing mouse models, e.g., the 5xFAD, to make research on these animals more reproducible between institutions.
Best Practices for Preclinical Testing
The center’s preclinical testing pipeline will evaluate biomarker changes after drug treatment. Because biomarker outcomes can be similar between mice and humans, they may translate better than behavioral testing, Masliah noted. “We don’t know what improvements in a water maze or an open field test equate to in a person,” he told Alzforum. At the NIA summits, scientists argued that preclinical testing has relied too much on behavioral outcomes as measures of efficacy, which may have created problems in translating drugs, Refolo added. Some researchers now eschew behavioral testing in mice altogether.
In addition, many previous preclinical studies did not use gold standard methods typically incorporated into clinical trials, such as randomization, blinding, and sample size calculations, Refolo said. The NIA has established the Alzheimer’s Disease Preclinical Efficacy Database to help promote best practices and improve reproducibility. The new center will further standardize preclinical methods, providing a template for others to follow. “We’re hoping for a sea change in the community,” Refolo said.—Madolyn Bowman Rogers
References
Conference Coverage Series Citations
Series Citations
News Citations
- New Initiative AMPs Up Alzheimer’s Research
- Knock-In Alzheimer’s Mice Catch on More Broadly in the Field
- Massive Icelandic Genome Analysis Offers Clues to Health and Disease, Including AD
- HAI—Sharper Curves: Revamping a Biomarker Staging Model
- Staging of Alzheimer’s, the Second: Neurodegeneration Does Not Equal Tauopathy
Research Models Citations
Paper Citations
- Ramanan VK, Risacher SL, Nho K, Kim S, Shen L, McDonald BC, Yoder KK, Hutchins GD, West JD, Tallman EF, Gao S, Foroud TM, Farlow MR, De Jager PL, Bennett DA, Aisen PS, Petersen RC, Jack CR Jr, Toga AW, Green RC, Jagust WJ, Weiner MW, Saykin AJ, Alzheimer’s Disease Neuroimaging Initiative (ADNI). GWAS of longitudinal amyloid accumulation on 18F-florbetapir PET in Alzheimer's disease implicates microglial activation gene IL1RAP. Brain. 2015 Oct;138(Pt 10):3076-88. Epub 2015 Aug 11 PubMed.
Other Citations
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
Certara
This is a very noble and timely initiative given the substantial translational issues between current transgene animal models based on familial mutations and the clinical reality with sporadic late-onset AD patients. Demonstrating an effect of a novel drug in preclinical models is helpful, maybe necessary, but probably not sufficient.
The challenge will be to ‘validate’ the new animal models. Face validity is certainly a possibility, but another possible avenue toward predictive validity is to check whether the animal models can reproduce the many clinical findings in patients under the same (or as close as possible) conditions of target engagement and co-medications that might affect cognitive readout. For instance, a substantial number of AD patients are prescribed drugs with an anticholinergic potential that might significantly affect clinical progression (Risacher et al., 2016).
Rather than looking at behavior and cognitive outcomes in preclinical models, one might maybe incorporate feedback on accessible and measurable objective biomarkers (plasma, CSF). This might have the additional benefit that we start to understand maybe why certain trials failed. However, such an approach necessitates having access to individual clinical data from trials, which is not a technical but more of a conceptual hurdle.
References:
Risacher SL, McDonald BC, Tallman EF, West JD, Farlow MR, Unverzagt FW, Gao S, Boustani M, Crane PK, Petersen RC, Jack CR Jr, Jagust WJ, Aisen PS, Weiner MW, Saykin AJ, Alzheimer’s Disease Neuroimaging Initiative. Association Between Anticholinergic Medication Use and Cognition, Brain Metabolism, and Brain Atrophy in Cognitively Normal Older Adults. JAMA Neurol. 2016 Jun 1;73(6):721-32. PubMed.
Indiana University
Indiana University School of Medicine
The Jackson Laboratory
The Jackson Laboratory
We thank Dr. Geerts for his comments regarding the MODEL-AD project. We also agree with his assessment regarding the importance of linking preclinical models to “accessible and measurable objective biomarkers (plasma, CSF, as well as RNA-Seq and brain imaging)” utilized in clinical studies. Notably, the MODEL-AD project will be doing just that, as we will be examining both fluid biomarkers (including Aβ, tau, p-tau, inflammatory cytokines/chemokines, among others), gene expression (RNA-Seq) as well as brain imaging biomarkers (MRI, Aβ-PET, Tau-PET, FDG-PET, among others) in the animal models that will be generated as part of the project. This will both allow us to assess the congruence between the animal models and human disease as well as establish potential biomarkers that could be utilized both in preclinical drug testing and ultimately in human clinical trials.
While we agree that these biomarkers will be extremely useful to determine the face validity and translatability of the animal models, ultimately it will also be key to examine various aspects of cognition in the animal models that move forward into preclinical drug testing. Given that specific memory impairments are the defining clinical feature observed in AD, it will ultimately be important to examine behavioral phenotypes in the new animal models and observe how these behavioral measures are linked (or not linked) to the biomarkers.
We look forward to engaging the research community as MODEL-AD gets started. We are working on a number of different venues and formats via which we can both inform the community as to our ongoing efforts and also get input/feedback from the larger research community, including manuscripts, presentations at national/international meetings, a Center web site, web-based conferences and others.
For more information, contact us at ModelAD@iupui.edu.
Make a Comment
To make a comment you must login or register.