Stress and Trauma: Shaken Brains, Shaken Lives
Quick Links
Athletes who suffer repeated concussions in high-impact sports such as boxing, football, and hockey are at risk for long-term brain damage. Some of them develop a degenerative condition known as chronic traumatic encephalopathy (CTE), also called dementia pugilistica (see ARF Live Discussion and ARF related news story). The condition can occur even in relatively young athletes. Hockey player Bob Probert, who died this year of heart failure at the age of 45, was recently found at autopsy to have CTE (see March 2 New York Times story). Probert was known as an “enforcer” due to his aggressive play and frequent fighting. The findings are sobering, given that Probert played in the modern era of helmet use, unlike previous CTE cases seen in hockey players from earlier eras.
The scope of the problem is great. The CDC estimates that more than a million Americans are treated for mild or moderate brain injuries each year, and about 200,000 of these injuries are sports related (see Morbidity and Mortality Weekly Report, 2007). However, because only about 10 percent of head injuries lead to a hospital visit, the actual number of traumatic brain injuries (TBIs) may be 10 times greater than this, the CDC noted. Researchers do not know how many of these TBIs are repeat injuries that increase the odds of developing a chronic condition, nor do they know how many concussions a brain can take before suffering long-term damage.
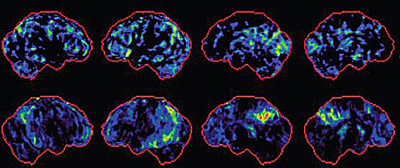
Brains with mild TBI (top row) show a similar pattern of changes to MCI brains (bottom row) by PET scan.Image credit: Elaine Peskind
Ann McKee, an expert on the pathology of sports-related head trauma at the Bedford VA Medical Center, Massachusetts, and Boston University, has probably seen more CTE brains than anybody. She runs brain banks at Bedford, Boston University, and the Center for the Study of Traumatic Encephalopathy at Boston University, among others, and has examined dozens of brains with CTE. CTE is “a unique disorder that is clearly associated with mild repetitive head injury,” McKee said, adding that it is “quite unlike any neurodegenerative disease I have ever studied.”
The disorder is primarily marked by deposits of tau and TAR DNA binding protein 43 (TDP-43), McKee said. Only about 40 percent of CTE brains she has looked at have Aβ deposits, and even when they are there, the plaques are diffuse and the amyloid load is smaller than in AD patients. “Aβ is definitely a characteristic of acute traumatic brain injury, but it may not persist into the chronic state,” said McKee, suggesting that Aβ deposition may be a phase reaction that is later cleared. This is similar to data from studies of severe TBI, which suggest that in that condition as well, Aβ deposits may be transient.
TDP-43 deposits also characterize amyotrophic lateral sclerosis (ALS) and some forms of frontotemporal dementia (FTD), while tau tangles mark Alzheimer’s disease and other subclasses of FTD. The tau tangles in CTE show abnormal phosphorylation similar to those seen in AD (see Schmidt et al., 2001). Although CTE shares features with other neurodegenerative disorders such as Parkinson’s, AD, and ALS, McKee said its overall presentation is distinctive.
Henrik Zetterberg at Sahlgrenska University Hospital in Molndal, Sweden, agrees that repeated head trauma causes a specific disorder. “I believe repeated brain trauma is more related to subcortical axonal injury than to an Alzheimer’s disease process per se,” he said. For instance, in one study that analyzed the cerebrospinal fluid (CSF) of boxers immediately after a match, Zetterberg and colleagues found elevated levels of biomarkers that indicate axonal injury, such as neurofilament light protein and total tau, but they did not see Alzheimer’s-like changes in Aβ or phosphorylated tau levels (see Zetterberg et al., 2006).
“This pattern looks more like what we see in cerebrovascular disease,” Zetterberg said. Because the brain cells that are most sensitive to hypoxia are myelin-producing oligodendrocytes, Zetterberg said, cerebrovascular disease damages subcortical myelinated thick-caliber axons, which may be the same axons damaged by repetitive head trauma. These axons connect association areas in the cortex and other brain regions. The resulting disorder gives a different clinical picture from Alzheimer’s, Zetterberg said. “The symptomatology is more slowness of thought and slowness of movement, parkinsonism combined with memory or cognitive problems.” One example of this presentation would be the boxer Muhammad Ali, who developed Parkinson’s-like symptoms after many years of boxing.
Nonetheless, Aβ may be involved as well, Zetterberg said. “Some processes in AD are activated by brain trauma,” he said. “Close to an injured axon, you have increased processing of β amyloid precursor proteins, so that more β amyloid is formed.” For example, an animal model of repetitive mild TBI demonstrated increased brain Aβ levels along with cognitive impairment (see Uryu et al., 2002). Patients with diffuse axonal injury have higher levels of Aβ42 in their brains than do people with contusions (see Marklund et al., 2009). However, David Brody of Washington University in St. Louis, Missouri, notes that this study did not compare Aβ42 levels with those in uninjured brains. Therefore, it is possible that both types of injuries lead to lowered extracellular Aβ levels compared to normal brains, which would be consistent with other studies, Brody said (see, e.g., Magnoni and Brody, 2010).
A puzzling fact is that, although CTE is primarily a tauopathy, its dementia seems to be modulated by ApoE genotype (see Jordan et al., 1997). One possibility, Sam Gandy at Mount Sinai Medical Center in New York City told ARF, is that blows to the head set off transient Aβ deposition, but the brain’s ability to efficiently clear away these deposits after each insult depends on ApoE genotype. Each round of Aβ deposition may also trigger tau, Gandy suggested, with tau pathology persisting and becoming self-perpetuating, even after Aβ is cleared. This may be similar to what happens in Alzheimer’s disease, Gandy noted. One theory holds that “the reason Aβ-lowering therapies fail in the clinic is because subjects with mild AD have already passed into a self-sustaining tau-dependent phase,” Gandy wrote to ARF.
Does CTE also occur in soldiers and marines exposed to repeated blasts from improvised explosive devices? These explosions create pressure waves that flex through bones and compress the brain, so the injuries may not be equivalent to impact concussions. McKee has just begun to look at donated brains from veterans exposed to repeated blast damage. She said that, although it is too early to be certain, there do appear to be large overlaps between these brains and what she sees in athletes with multiple concussions. She expects there will be some differences as well. Football players may get around 1,000 sub-concussive hits per season from playing on the line, McKee said, and it is not yet clear how that corresponds to receiving shock waves from one or several explosions. It does appear that blasts have to be strong enough to cause disorientation in order to be damaging: A recent study showed that simply being near large weapon blasts, such as the firing of artillery shells, did not create any changes in CSF biomarkers (see Blennow et al., 2011). For the latest data on what blast exposure does to brains, read Part 4.—Madolyn Bowman Rogers.
This is Part 3 of a six-part series. See also Part 1, Part 2, Part 4, Part 5, Part 6.
References
Webinar Citations
News Citations
- TDP-43 and Tau Entangle Athletes’ Nerves in Rare Motor Neuron Disease
- Stress and Trauma: Blast Injuries in the Military
- Stress and Trauma: New Frontier Lures Alzheimer’s Researchers
- Stress and Trauma: Aβ’s Mysterious Role in Severe Brain Injury
- Stress and Trauma: The Puzzle of Post-traumatic Stress Disorder
- Stress and Trauma: Tackling Post-traumatic Stress Disorder
Paper Citations
- Nonfatal traumatic brain injuries from sports and recreation activities--United States, 2001-2005. MMWR Morb Mortal Wkly Rep. 2007 Jul 27;56(29):733-7. PubMed.
- Schmidt ML, Zhukareva V, Newell KL, Lee VM, Trojanowski JQ. Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer's disease. Acta Neuropathol. 2001 May;101(5):518-24. PubMed.
- Zetterberg H, Hietala MA, Jonsson M, Andreasen N, Styrud E, Karlsson I, Edman A, Popa C, Rasulzada A, Wahlund LO, Mehta PD, Rosengren L, Blennow K, Wallin A. Neurochemical aftermath of amateur boxing. Arch Neurol. 2006 Sep;63(9):1277-80. PubMed.
- Uryu K, Laurer H, McIntosh T, Praticò D, Martinez D, Leight S, Lee VM, Trojanowski JQ. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J Neurosci. 2002 Jan 15;22(2):446-54. PubMed.
- Marklund N, Blennow K, Zetterberg H, Ronne-Engström E, Enblad P, Hillered L. Monitoring of brain interstitial total tau and beta amyloid proteins by microdialysis in patients with traumatic brain injury. J Neurosurg. 2009 Jun;110(6):1227-37. PubMed.
- Magnoni S, Brody DL. New perspectives on amyloid-beta dynamics after acute brain injury: moving between experimental approaches and studies in the human brain. Arch Neurol. 2010 Sep;67(9):1068-73. PubMed.
- Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, Gandy S. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA. 1997 Jul 9;278(2):136-40. PubMed.
- Blennow K, Jonsson M, Andreasen N, Rosengren L, Wallin A, Hellström PA, Zetterberg H. No neurochemical evidence of brain injury after blast overpressure by repeated explosions or firing heavy weapons. Acta Neurol Scand. 2011 Apr;123(4):245-51. PubMed.
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
National Institute on Aging, NIH
Worth thinking about, too, in this context, are the shaken babies who survive and the impact that such trauma will have on their neural development.
Make a Comment
To make a comment you must login or register.