Do Tau Fibrils Form Via Droplets?
Quick Links
How do tau fibrils develop? In the July 19 Structure online, scientists led by Yoshiyuki Soeda and Akihiko Takashima at Gakushuin University, Tokyo, proposed that the protein first condenses into droplets, then folds into insoluble β sheets. When the authors forced mutant human tau in a mouse cell line to come together, it underwent liquid-liquid phase separation (LLPS), forming spherical globs in the cytoplasm.
- Mutant human tau jammed together in cells forms liquid droplets.
- Snipping tau’s N-terminus promotes formation of β sheets within droplets.
- Truncated tau is common in aged brain, hinting at biological relevance.
This tau was highly phosphorylated and oligomeric, but also dissolvable by alcohol or detergent. Once the scientists deleted the N-terminal region, however, tau became prone to aggregate. Insoluble β sheets capable of seeding fibrils formed inside droplets. N-terminal cleavage and LLPS may be intermediate steps on the way to neurofibrillary tangles, the authors suggested.
Ben Wolozin at Boston University praised the study’s excellent use of common cell biology tools to gain new insights into tau aggregation. “[The paper] conveys an important difference between potentially functional tau oligomerization—which proceeds through LLPS—and pathological tau oligomerization and aggregation, which correlates with formation of insoluble, fibrillar tau,” he wrote (comment below).
Previous studies had linked liquid droplets of tau to the production of toxic forms, but haven’t answered the question of how this happens even in cell culture, much less the aging human brain (Aug 2017 news; Wegmann et al., 2018; Kanaan et al., 2020). Moreover, some scientists have found that tau can aggregate via other routes, without first forming droplets (Aug 2017 conference news; Apr 2024 news).
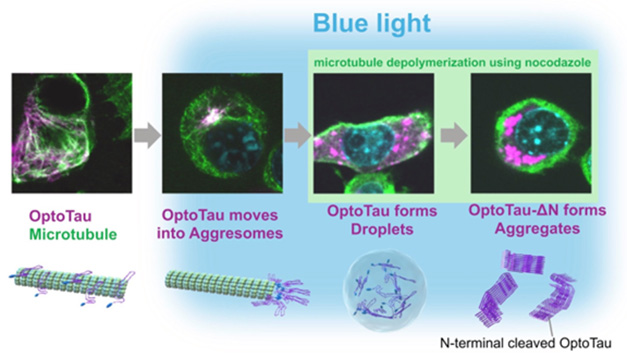
Stages of Fibrillization? When mutant human tau (purple) fused to a light-responsive protein (OptoTau, left) is induced to congregate via blue light, it accumulates in aggresomes of neuroblastoma cells (middle left). If aggresome transport is blocked by microtubule (green) disassembly, tau droplets appear in the cytoplasm (middle right). When this condensed tau lacks its N-terminus, it folds into β sheets (far right). Nucleus is blue. [Courtesy of Soeda et al., Structure.]
To gain mechanistic insight, Takashima’s group turned to a highly artificial system. First author Soeda expressed human P301L tau, which aggregates readily, in a mouse neuroblastoma cell line. Tau was fused to a second protein, the plant photoreceptor arabidopsis cryptochrome 2 (CRY2). When the authors shone blue light onto the cells, CRY2 oligomerized, bringing tau monomers close to each other. This tool is commonly used to promote protein aggregation, and it accelerated tau deposition, i.e., clumps of hyperphosphorylated, oligomeric tau accumulating near the nucleus.
The authors believed these clumps might represent aggresomes, protein snarls that build up when a cell’s degradation machinery is overwhelmed. In such cases, cells transport indigestible protein bits along their microtubules and sequester them near the nucleus. Supporting this idea, when the authors added the microtubule buster nocodazole to the cell media, tau no longer accumulated near the nucleus. Instead, it appeared in round droplets throughout the cytoplasm.
The droplets gradually disappeared when the authors turned off the blue light, meaning the tau remained soluble. In addition, adding the alcohol 1,6-hexanediol to the cells dispersed the droplets, as it does for other protein droplets. Likewise, when the authors treated cell lysates with the detergent Sarkozy, tau clumps broke up, again showing it contained no insoluble β sheets.
To spark fibrillization, the authors snipped off tau’s N-terminus. Insoluble tau deposits tend to lack this tail, suggesting its presence may hinder β-sheet formation (Dec 2020 news). Sure enough, the truncated tau/CRY2 chimera made eight times as many oligomers after exposure to blue light as did full-length tau/CRY2. Moreover, the tau clumps now contained β sheets. Droplets were no longer spherical, and they bound the fibril marker thioflavin S. They also stayed stable after the blue light was switched off, and resisted degradation by 1,6-hexanediol and sarkosyl.
These tau aggregates were not long enough to be considered fibrils, the authors found. They did, however, seed aggregation in solutions of monomeric tau, confirming the presence of β sheets.
The authors believe the findings are relevant to what happens in the brain. N-terminally truncated tau increases with age in tau transgenic mice, suggesting this form could spur aggregation (Matsumoto et al., 2015). “Our findings support a model whereby P301L tau clustering is accumulated in aggresomes initially, and disruption of the aggresomal process leads to tau aggregation through LLPS, ultimately forming a tau seed,” they wrote.—Madolyn Bowman Rogers
References
News Citations
- More Droplets of Tau
- Monomeric Seeds and Oligomeric Clouds—Proteopathy News from AAIC
- Tau Toggling Peptides: One Seeds Fibrils; the Other Dismantles Them
- Mounting Modifications Move Tau Toward Aggregation in Alzheimer’s Brain
Mutations Citations
Paper Citations
- Wegmann S, Eftekharzadeh B, Tepper K, Zoltowska KM, Bennett RE, Dujardin S, Laskowski PR, MacKenzie D, Kamath T, Commins C, Vanderburg C, Roe AD, Fan Z, Molliex AM, Hernandez-Vega A, Muller D, Hyman AA, Mandelkow E, Taylor JP, Hyman BT. Tau protein liquid-liquid phase separation can initiate tau aggregation. EMBO J. 2018 Apr 3;37(7) Epub 2018 Feb 22 PubMed.
- Kanaan NM, Hamel C, Grabinski T, Combs B. Liquid-liquid phase separation induces pathogenic tau conformations in vitro. Nat Commun. 2020 Jun 4;11(1):2809. PubMed.
- Matsumoto SE, Motoi Y, Ishiguro K, Tabira T, Kametani F, Hasegawa M, Hattori N. The twenty-four KDa C-terminal tau fragment increases with aging in tauopathy mice: implications of prion-like properties. Hum Mol Genet. 2015 Nov 15;24(22):6403-16. Epub 2015 Sep 15 PubMed.
Further Reading
Primary Papers
- Soeda Y, Yoshimura H, Bannai H, Koike R, Shiiba I, Takashima A. Intracellular tau fragment droplets serve as seeds for tau fibrils. Structure. 2024 Oct 3;32(10):1793-1807.e6. Epub 2024 Jul 19 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.