T Cells in the Brain Goad Microglia to Muddle Myelin
Quick Links
The idea that infiltrating T cells contribute to a neurodegenerative environment in aging and in Alzheimer’s disease has gotten another boost. In the June 27 Nature Neuroscience, scientists led by Mikael Simons, Technical University Munich, reported that in mouse models of amyloidosis, CD8+ T cells stir a subset of interferon-γ-responsive microglia, which, in turn, hinder oligodendrocytes, leaving myelin to unfold, split, and degenerate. Knocking down the T cells or the microglia reverses the damage, while leaving amyloid untouched. The work indicates that the adaptive immune system may trigger the degradation of myelin and white matter seen in AD and other neurodegenerative diseases.
- In AD, CD8+ T cells infiltrate the brain and release interferon-γ.
- This stirs highly phagocytic microglia that attack myelin.
- The microglia cripple oligodendrocytes compromising myelin repair.
“This work provides compelling evidence that CD8+ T cells and microglia interactions are pivotal in the myelin pathology observed in Alzheimer’s disease,” wrote Mehdi Jorfi and Rudolph Tanzi of Massachusetts General Hospital in Boston.
Xiaoying Chen and David Holtzman at Washington University in St. Louis praised the study. “These results beautifully highlight the specific importance of microglia-T cell interactions in white-matter damage, an important target for preserving and maintaining normal axonal function and cognition,” they wrote (comments below).
Myelin sheaths that insulate neurons in the white matter degrade as a part of normal aging and, to a greater extent, during neurodegenerative disease. Scientists do not know why this happens, but with age, oligodendrocytes—the cells responsible for maintaining myelin—morph into states associated with myelin damage and disease, including those producing the serine protease inhibitor Serpina3n (Jan 2020 news; Fissolo et al., 2021; Kenigsbuch et al., 2022).
To better understand any role these oligodendrocytes play in myelin degradation during AD, co-first authors Shreeya Kedia of TUM and Hao Ji at Ludwig-Maximilian-University, also in Munich, turned to mouse models of amyloidosis. First, the scientists analyzed brain tissue from 5xFAD and APPNL-G-F mice when their amyloid plaque load had plateaued, at 10 and 12 months old, respectively. They spotted Serpina3n-positive oligodendrocytes and oligodendrocyte precursor cells (OPCs) in the white and gray matter, whereas no, or very few, of these cells showed up in age-matched wild-type mice.
Kedia and colleagues found the Serpina3n oligodendrocytes had swelled with myelin basic protein (MBP), and their processes had fragmented, suggesting they were overwhelmed with myelin. Scanning electron microscopy showed abnormal myelin structures, including outfolds, splits, fragments, and even an excess in places (image below).
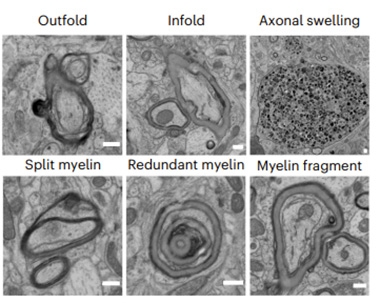
Myelin Mayhem. White and gray matter of 10-month-old 5xFAD mice had various kinds of myelin damage and axons swollen with myelin. [Courtesy of Kedia et al., Nature Neuroscience, 2024.]
Simons thinks that OPC proliferation hints at a regenerative response to neuroinflammation that fails because maturing oligodendrocytes malfunction and die. What would cause the inflammation? In the 10-month-old 5xFAD and 12-month-old APPNL-G-F mice, the authors found an infiltration of CD8+ T cells in the white and gray matter. Did these mess with the oligodendrocytes and the myelin? Injecting an anti-CD8 antibody into the abdomens of 6-month-old 5xFAD mice—the age when CD8+ T cells appear in gray matter—led to two-thirds fewer Serpina3n oligodendrocytes in the cortex and one-third fewer myelin abnormalities than in controls six weeks later (image below). The opposite was true in 5xFAD animals injected with anti-PD-1 and anti-CTLA-4 antibodies, which release the brakes on immune checkpoints—these mice cranked out more CD8+ T cells and myelin damage jumped about 50 percent. Likewise, APPNL-G-F mice crossed with Rag1 knockout mice, which can’t make B and T cells, had normal myelin. Taken together, these results suggest that the T cells exacerbate oligodendrocyte failure and myelin abnormalities.
Notably, the amyloid load remained unchanged whether CD8+ T cells were depleted or multiplied, suggesting that myelin and amyloid pathology are independent.
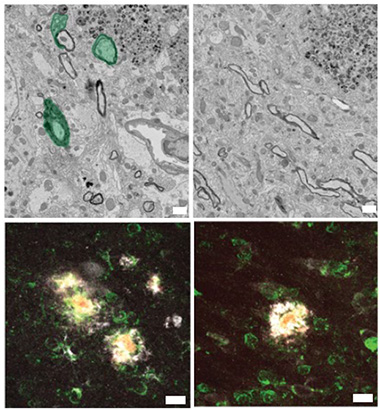
Meddling T Cells. Scanning electron microscopy found abnormal myelin (green, top left) in the brains of 5xFAD mice. Oligodendrocytes expressed Serpina3n (white, middle left). When given an anti-CD8 antibody (right column), myelin returned mostly to normal, and the disease-associated oligodendrocytes diminished. [Courtesy of Kedia et al., Nature Neuroscience, 2024.]
How did the CD8+ T cells damage myelin? The scientists suspected microglia were involved. Single-cell RNA sequencing teased out nine types of microglia in 5xFAD mice, including homeostatic, interferon-responsive, and disease-associated (DAM). A subset of the latter, expressing many MHC-II genes, caught the researchers' attention because these cells are highly active and phagocytic. They believe these are in an advanced stage of activation. APPNL-G-F/Rag1 KO mice, and 5xFAD mice given the anti-CD8 antibody had 70 percent fewer MHC-II-positive DAMs than APPNL-G-F and 5xFAD controls. On the other hand, 5xFAD mice given immune checkpoint blockers had twice as many CD8+ T cells, and twice as many MHC-II-positive DAMs.
In 5xFAD brain slices, these MHC-II-positive DAMs were stuffed with MBP, and, when the cells were added to brain slices from wild-type mice, they attacked and engulfed the protein there. Within 5xFAD brain slices, cells next to CD8+ T cells turned up expression of MHC-II genes. Similarly, in hippocampal tissue from people who had had AD, 45 percent of CD8+ T cells were within 50 microns of MHC-II-positive myeloid cells.
The authors think CD8+ T cells stir microglia via interferon-γ (IFNg) because the leukocytes crank out this cytokine, and it induces MHC-II expression (Bhat et al., 2017; Steimle et al., 1994). Indeed, the MHC-II-positive microglia turned on interferon-responsive genes. In vitro, IFNγ roused wild-type microglia to express MHC-II, while injected into 5xFAD mice, it almost doubled MHC-II-positive microglia.
In contrast, giving 5xFAD mice the IFN pathway blocker baricitinib for 12 weeks halved myelin abnormalities even though CD8+ T cell numbers stayed elevated. The baricitinib-treated animals escaped from a Barnes maze faster than controls, as did mice given the anti-CD8 antibody. The data indicate that these myelin deficits have physiological consequences and align with the idea that myelination supports memory (Feb 2020 news).
On that note, Alma Mohebiany and Renzo Mancuso, VIB-Center for Molecular Neurology, Antwerp, Belgium, were intrigued. “Further studies are needed to determine whether CD8 T cells play a region-specific role in how they promote or prevent pathology,” they wrote. “However, there are still interesting implications for the treatment of AD, in that blocking inflammation-induced microglia activation could slow down of learning or memory deficits through preservation of white matter.” (Comment below.)
“Overall, [these results] suggest that targeting the interactions between CD8+ T cells and microglia, or modulating specific interferon pathways, could open new avenues for delaying or halting the progression of AD,” wrote Jorfi and Tanzi.—Chelsea Weidman Burke
References
News Citations
- Human and Mouse Microglia React Differently to Amyloid
- New Myelin Makes Memories, but Supply Wanes with Age
Research Models Citations
Paper Citations
- Fissolo N, Matute-Blanch C, Osman M, Costa C, Pinteac R, Miró B, Sanchez A, Brito V, Dujmovic I, Voortman M, Khalil M, Borràs E, Sabidó E, Issazadeh-Navikas S, Montalban X, Comabella Lopez M. CSF SERPINA3 Levels Are Elevated in Patients With Progressive MS. Neurol Neuroimmunol Neuroinflamm. 2021 Mar;8(2) Print 2021 Mar PubMed.
- Kenigsbuch M, Bost P, Halevi S, Chang Y, Chen S, Ma Q, Hajbi R, Schwikowski B, Bodenmiller B, Fu H, Schwartz M, Amit I. A shared disease-associated oligodendrocyte signature among multiple CNS pathologies. Nat Neurosci. 2022 Jul;25(7):876-886. Epub 2022 Jun 27 PubMed.
- Bhat P, Leggatt G, Waterhouse N, Frazer IH. Interferon-γ derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. 2017 Jun 1;8(6):e2836. PubMed.
- Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994 Jul 1;265(5168):106-9. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Kedia S, Ji H, Feng R, Androvic P, Spieth L, Liu L, Franz J, Zdiarstek H, Anderson KP, Kaboglu C, Liu Q, Mattugini N, Cherif F, Prtvar D, Cantuti-Castelvetri L, Liesz A, Schifferer M, Stadelmann C, Tahirovic S, Gokce O, Simons M. T cell-mediated microglial activation triggers myelin pathology in a mouse model of amyloidosis. Nat Neurosci. 2024 Aug;27(8):1468-1474. Epub 2024 Jun 27 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.