Viral Vector Targets Transferrin Receptor to Deliver GBA1 to Brain
Quick Links
Over the last three decades, scientists have devised molecular shuttles that slip enzymes, antibodies, and other molecules into the central nervous system (CNS) by attaching them to the transferrin receptor, which ferries the cargo across the blood-brain barrier. Now, researchers led by Benjamin Deverman at the Broad Institute in Cambridge, Massachusetts, have piggy-backed on that strategy to deliver genes into the brain. In the May 16 Science, they reported proof-of-concept data, showing that a transferrin-binding motif boosted delivery of adeno-associated virus 9 (AAV9) into the brain 30-fold. The virus, carrying the GBA1 gene, produces seven times as much β-glucocerebrosidase in the mouse brain as did a control virus. Glucocerebrosidase deficiency causes Gaucher’s disease, a rare childhood neurodegenerative disorder, and mutations in the enzymes increase the risk of Parkinson’s disease. Other gene therapies aim to boost the enzyme’s activity (Jul 2018 news).
- Transferrin receptor on endothelial cells can shuttle large molecules into the brain.
- A virus targeting the receptor sneaks genes into the brain.
- It boosts viral delivery 30-fold.
“A critically needed improvement for CNS gene therapy is the characterization of vectors able to efficiently cross the blood-brain barrier after administration in the bloodstream,” wrote Olivier Danos of Maryland-based Regenxbio (comment below). “This paper is exciting because it shows, through a series of impeccably executed studies, that some of the current limitations of the technology can be solved.”
Joy Zuchero of Denali Therapeutics in South San Francisco called the strategy clever. “This elegant paper represents a significant advancement and opens the door for an exciting new approach in the area of CNS gene delivery,” she wrote (comment below). Denali targets the transferrin receptor to deliver a TREM2 antibody, the progranulin protein, and iduronate-2-sulfatase, an enzyme defective in Hunter syndrome, into the brain (Jan 2023 news; Apr 2023 news; Jun 2023 news). Roche uses a similar strategy to deliver trontinemab, a version of their anti-amyloid antibody, gantenerumab (Mar 2021 conference news).
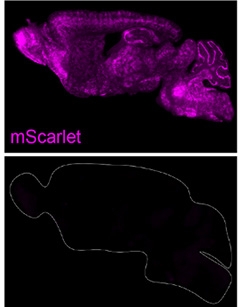
Seeing Scarlet. An AAV9 targeting the transferrin receptor delivered the gene for mScarlet fluorescent protein throughout the brain (top), but an AAV9 carrying the same gene could not (bottom). [Courtesy of Huang et al., Science, 2024.]
Widely used for gene therapies, AAVs come in subtypes that selectively infect certain tissues or cells. AAV9, for one, crosses the BBB and is commonly used for CNS therapies, such as the FDA-approved spinal muscular atrophy treatment Zolgensma (Nov 2019 conference news). Deverman and colleagues thought to enhance this BBB penetration by creating a virus that can be shuttled by the transferrin receptor (TfR). Co-first authors Qin Huang and Ken Chan modified AAV9 viruses, randomly mutating a stretch of seven amino acids within the viral capsid sequence, then screening the tens of millions of viral variants for binding to human TfR. One AAV, called BI-hTFR1 after the Broad Institute, bound tightest to the receptor.
Would this Trojan horse work in vivo? The scientists loaded BI-hTFR1 viruses with luciferase genes, then added them to primary and cultured human brain endothelial cells. These cells shunt transferrin from the blood into the brain parenchyma. BI-hTFR1 delivered 35- and 55-fold more luciferase into the primary and cultured cells, than did AAV9.
Could BI-hTFR1 cross the BBB in vivo? Huang and Chan intravenously injected BI-hTFR1 or AAV9 carrying the gene for the mScarlet fluorescent protein into mice that had the human TfR gene knocked in. Three weeks later, cells glowed throughout the brains of animals injected with BI-hTFR1 but not in those given AAV9 (image above). Across the cortex, striatum, and thalamus, about one-quarter of the oligodendrocytes, half the neurons and almost all the astrocytes expressed mScarlet, measured via fluorescence and immunohistochemistry. There was little expression in microglia. “It will be of great value to continue to evolve the capsid for microglia targeting as well, given this cell type's role in a number of genetically linked neurodegenerative diseases,” wrote Zuchero.
The authors anticipate that BI-THFR1 could better deliver therapeutic genes into the brain. To test this, Huang and Chan injected AAVs carrying GBA1 into the veins of human TfR knock-in mice. Three weeks later, 30 times as much BI-hTFR1 viral DNA was in the animals’ brains as AAV9 DNA. BI-hTFR1-treated mice widely expressed GBA1 in the brain, mainly in neurons and astrocytes (image below). Their brains had 6.7 times more β-glucocerebrosidase activity than did AAV9-treated mice, suggesting that higher gene expression translated into more enzyme.
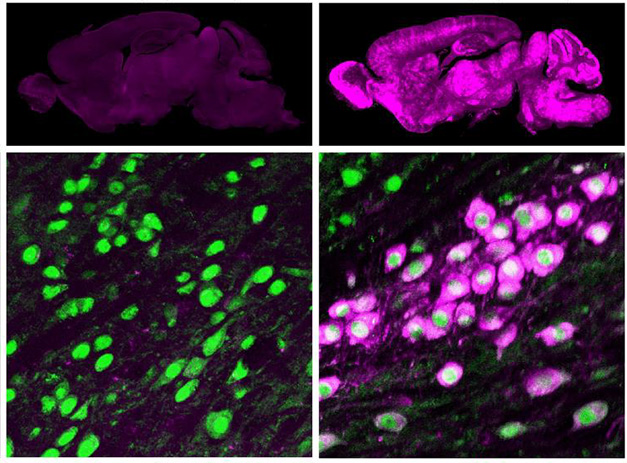
Transferring GBA1. AAV9-GBA1 at a dose of 1 x 1014 viral genomes/kg (purple, left) barely infected any neurons (green) in TfR knock-in mice. However, BI-hTFR1 given at the same dose (right) infected neurons throughout the brain. [Courtesy of Huang et al., Science, 2024.]
“It is promising to see successful delivery of GBA1 using BI-hTfR1 as a proof of concept,” Zuchero wrote. Deverman has made the plasmid for BI-hTFR1 available for other researchers through Addgene. He said his group is creating a gene therapy for an adult neurodegenerative disease using BI-hTFR1. New York-based Apertura Gene Therapy, a company he co-founded, has two programs based on the AAV. Deverman would not say which diseases they are targeting.—Chelsea Weidman Burke
References
News Citations
- Gene Therapy Treats Mouse Models of Gaucher’s Disease
- Ferried Into Brain, TREM2 Antibody Stirs Microglia
- Pumping Up Progranulin: Scientists Show New Efforts to Get It Done
- Treatment for Lysosomal Storage Disorder Lowers NfL
- Shuttle Unloads More Gantenerumab Into the Brain
- Time to Try Again: Gene-Based Therapy for Neurodegeneration
Therapeutics Citations
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Huang Q, Chan KY, Lou S, Keyes C, Wu J, Botticello-Romero NR, Zheng Q, Johnston J, Mills A, Brauer PP, Clouse G, Pacouret S, Harvey JW, Beddow T, Hurley JK, Tobey IG, Powell M, Chen AT, Barry AJ, Eid FE, Chan YA, Deverman BE. An AAV capsid reprogrammed to bind human Transferrin Receptor mediates brain-wide gene delivery. bioRxiv. 2023 Dec 22; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
REGENXBIO
Gene transfer vectors derived from Adeno-Associated Virus (AAV) are the tools of choice for gene therapy (and gene editing) in the central nervous system. This paper is exciting because it shows, through a series of impeccably executed studies, that some of the current limitations of the technology can be solved. It considerably broadens the scope of potential applications, including in Alzheimer’s disease.
A critically needed improvement for CNS gene therapy is the characterization of vectors able to efficiently cross the blood-brain barrier after administration in the bloodstream. Several candidates now exist, but the Deverman group has introduced a new—and successful—twist in this quest.
AAV vectors enter their target cells through interactions between their capsid protein and cell surface proteins and sugars. The effective delivery of the therapeutic payload then involves multiple cellular proteins. Naturally occurring AAVs have evolved their capsids to adapt to the molecular partners found in their host cell of choice and thus, different AAV subtypes may display a particular cellular host range. Understanding this has led to the idea that the AAV capsid can be further evolved through genetic engineering towards a highly precise, and possibly programmable, host-cell specificity.
To do this, highly complex libraries of AAV variants are generated by randomizing surface loops on the AAV capsids. Rounds of selection in vivo then take place, eventually leading to the identification of candidates with an optimized fit for a tissue, cell type, or route of administration. However, it has become quickly evident that many candidates obtained in one species do not perform well in another, which creates a problem of predictability that seriously impairs drug development.
To mitigate this, the Deverman group first screened the AAV library directly against a cellular partner of interest through in vitro panning. The partner they have chosen is, not too surprisingly, the apical domain of the human Transferrin receptor, which is already used to ferry antibodies and enzymes across the vascular endothelial cells forming the blood-brain barrier. The approach yields very potent candidates with high affinity for the BBB cells and a 10- to 50-fold increase in their ability to deliver therapeutic genes into the brain. These are significant improvements over AAV9, the parent capsid from which the library was constructed. AAV9 is used in Zolgensma, an approved drug for gene therapy of spinal muscular atrophy.
This work is an important step forward but certainly does not stop here. We now need to take care of the other hurdles that limit access of AAV vectors to the CNS, finding capsids that do not accumulate in the liver and are less effectively eliminated by the immune system are required. In this respect the direct panning of libraries against relevant proteins deployed in the paper may be relevant.
Others are exploring a different avenue to target AAV capsids to the CNS by directly coupling an otherwise inactive capsid to antibodies that will drive it to the target. This approach is more modular and could have advantages for drug development, including the possibility of studying drug candidates in multiple species.
Denali Therapeutics
Broadening the utilization of AAVs to treat CNS diseases is an exciting area of therapeutic development, but one that faces many challenges for clinical translation. This paper makes significant progress toward one such challenge in this field, which is translatability of successful brain-penetrating capsids from preclinical species to humans. It is a clever approach to combine decades of research and mechanistic understanding of TfR-enabled brain delivery with application to AAV gene delivery.
The enhanced brain tropism compared to other peripheral tissues is another major advance compared to other common AAVs. It is promising to see successful delivery of GBA1 to the brain using BI-hTfR1 as a proof of concept, and at significantly lower doses of virus than typically used for delivery to the CNS. Although many of the major cell types of the brain were successfully transduced, it will be of great value to continue to evolve the capsid for microglia targeting as well, given the role these cells play in a number of genetically linked neurodegenerative diseases. Longer-term studies, and in higher species, will be important to better understand whether TfR-targeting AAVs introduce additional safety and/or immunogenicity challenges.
Nonetheless, this elegant paper represents a significant advance and opens the door for an exciting new approach in the arena of CNS gene delivery.
Uppsala Universitet
Uppsala University
The study by Huang and colleagues describes the evaluation of an AAV vector engineered to actively enter the brain via transferrin receptor (TfR)-mediated transcytosis. This route to enter the brain is well-established for biologics, such as antibodies, with Roche’s brain shuttle (Trontinemab) as an example of an AD-related molecule that is presently evaluated in clinical trials, with promising results. Previously developed brain-targeted AAV vectors have been selected based on efficient brain uptake with an unknown mechanism of action. Here, the authors took the approach to first identify a mechanism (TfR-mediated transcytosis) and then select AAVs from a library with variations in a seven-amino-acid stretch of the capsid surface protein.
The authors convincingly demonstrate that the TfR-AAV enters the brain of a mouse with the apical domain of the human TfR knocked into its genome. The AAV entered the brain parenchyma and, to a large degree, also various brain cells. Importantly, they also show that the AAV can deliver cargo, which in turn can elicit a pharmacological response through increased activity of glucocerebrosidase in both the brain and CSF. However, as the authors point out, the present study did not demonstrate the use of this vector to “correct” a genetic component in a model of disease. It will be interesting to follow future development in this regard and compare it to presently used vectors for gene therapy.
In the field of antibody- or enzyme-based biologics that use TfR-mediated brain entry, the mode of TfR binding has been thoroughly studied and debated. Variables such as affinity, mono- or multivalent binding, or protein architecture have been shown to affect both brain transport and the safety profile of the molecule. An AAV capsid that supposedly carries multiple TfR-binding proteins on its relatively large surface can probably be expected to bind TfR with several binding sites simultaneously, which raises the question of whether this high TfR avidity could induce cell-surface TfR clustering, leading to internalization and degradation rather than transcytosis and recycling of the receptor. Indeed, although similar to the AAV9 control, almost 50 percent of endothelial cells were transduced by the TfR-AAV in the human TfR knock-in mouse, with only 10 to 15 percent transduced in the WT mouse used as a negative control. Another aspect of TfR binding is that it can affect blood pharmacokinetics through interaction with TfR on blood cells and peripheral organs. The present study does not report blood data, making it difficult to judge whether there is interaction with TfR on blood cells. It should be noted that interactions with TfR on reticulocytes in the bloodstream can, under certain circumstances, cause hemolysis.
Finally, off-target effects are often discussed in relation to gene therapy. The authors claim that one advantage of TfR-targeted AAV delivery is the brain endothelial-specific expression of TfR. However, TfR is, in fact, expressed in many other organs, and, although the TfR-AAV was indeed shown to be present at much higher concentrations in the brain compared to the control AAV9, it did not escape uptake in other organs. For example, the biodistribution experiment showed massive amounts of viral genome in the liver. Thus, although impressive in its capacity to deliver cargo to the brain, a certain amount of caution is probably warranted in future studies of this novel virus vector system.
Make a Comment
To make a comment you must login or register.