Two Paths for TREM2-Positive Microglia: DAM or Senescence?
Quick Links
Numerous studies have cast TREM2-expressing microglia as good guys in the Alzheimer’s brain, but new research suggests some play a darker role. In the April 18 Nature Neuroscience, researchers led by Michal Schwartz and Valery Krizhanovsky at the Weizmann Institute of Science, Rehovot, Israel, reported that some TREM2-positive microglia in an amyloidosis mouse model become senescent. Eliminating these cells cooled neuroinflammation and improved memory, indicating they harm the brain. Like disease-associated microglia (DAM), senescent microglia require TREM2, with few forming in TREM2 knockouts. Senescent microglia could be distinguished from DAM by their protein signature, but not easily by gene expression, explaining why they have been overlooked in previous transcriptomic studies.
- Senescent microglia in amyloidosis mice express high levels of TREM2.
- They have distinct protein profiles from disease-associated microglia (DAM).
- The two TREM2 subtypes are hard to distinguish in transcriptomic studies.
“This study suggests that TREM2 displays a dual activity in microglia, which should be carefully considered when contemplating TREM2 as a therapeutic target,” the authors noted.
Marco Colonna at Washington University School of Medicine, St. Louis, called the paper insightful, and said it raises several mechanistic questions for future study, such as how TREM2 expression leads to senescence. “Additionally, it underscores the value of integrating protein data alongside transcriptional data to account for potential disparities,” Colonna wrote (comment below).
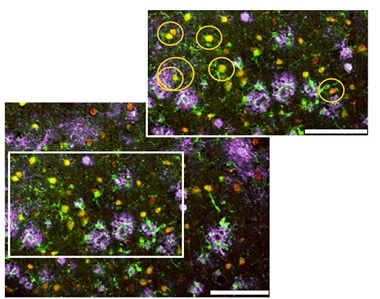
Close Yet Aloof. In the 5xFAD mouse cortex, senescent microglia (red) occur near (gold circles) but do not surround plaques (pink) as do healthy microglia (green). [Courtesy of Rachmian et al., Nature Neuroscience.]
Diego Gómez-Nicola at the University of Southampton, U.K., had previously reported that DAM could turn senescent (Jun 2021 news). Another study found senescent microglia that were unable to transition into a DAM state in amyloidosis mice (May 2023 news). Still, little is known about these cells and how they form.
To further characterize senescent microglia, Schwartz and colleagues focused on their protein profile, rather than gene expression. First author Noa Rachmian isolated microglia from the brains of 2-year-old 5xFAD mice, then incubated the cells with antibodies labeled with heavy-metal ions. These antibodies were directed against 33 senescence factors and key microglial proteins. The cells were then shot through a mass spectrometer one at a time to quantify each targeted protein.
To the authors’ surprise, microglia that expressed senescent markers also expressed TREM2, ApoE, and other proteins characteristic of activated microglia. However, they had a distinct profile from DAM, expressing some homeostatic proteins as well, such as TMEM119, P2RY12, and CX3CR1. Moreover, senescent microglia expressed about twice as much TREM2 as did DAM, again indicating they represent a distinct cell population. In immunostained cortex, the senescent cells appeared near amyloid plaques, but did not surround them as do DAM (image at right).
TREM2 expression correlated with the amount of senescent proteins, causing the authors to wonder if TREM2 might help trigger this cell state. Supporting this, when the authors crossed 5xFAD mice with TREM2 knockouts, the offspring accumulated less than half as many senescent microglia, amounting to about 7 percent of all microglia, compared with 18 percent in 5xFAD mice.
Gómez-Nicola said the dependence of senescent microglia on TREM2 is a new finding, and speculated it might be linked to TREM2’s effects on microglial survival and proliferation (Feb 2015 news; Apr 2017 conference news). “Senescent microglia may be more susceptible to the evolving AD-like pathology, therefore becoming more dependent on mechanisms of survival,” Gómez-Nicola wrote (comment below).
Why have TREM2-positive senescent microglia not been widely reported before? Most studies characterizing microglial subtypes have been transcriptional. When the authors reanalyzed previous microglial RNA-Seq datasets, they detected a population of senescent microglia, but found they were hard to discern from DAM. Protein signatures can differ from gene-expression profiles due to post-translational modifications, protein stability, and the different sensitivities of the methods, they noted.
Senescent cells are typically harmful, and that was the case here as well. The authors injected 11-month-old 5xFAD mice with the senolytic drug ABT-737 for three days, then analyzed their brains two weeks later. The number of senescent microglia had been cut nearly in half, while DAM were unchanged. Inflammatory cytokines were suppressed, while the mice’s ability to recognize a new object bounced back to that of wild-types. Another senolytic combination, dasatinib plus quercetin, is being tested in AD patients (Dec 2021 news; Gonzales et al., 2023).—Madolyn Bowman Rogers
References
News Citations
- DAMned to Death? Microglia May Proliferate to Senescence
- When Autophagy Stops, Microglia Sour into Senescence
- TREM2 Buoys Microglial Disaster Relief Efforts in AD and Stroke
- New Evidence Confirms TREM2 Binds Aβ, Drives Protective Response
- Young ApoE4 Carriers Have Reversed AD Proteomic Signature
Research Models Citations
Therapeutics Citations
Paper Citations
- Gonzales MM, Garbarino VR, Kautz TF, Palavicini JP, Lopez-Cruzan M, Dehkordi SK, Mathews JJ, Zare H, Xu P, Zhang B, Franklin C, Habes M, Craft S, Petersen RC, Tchkonia T, Kirkland JL, Salardini A, Seshadri S, Musi N, Orr ME. Senolytic therapy in mild Alzheimer's disease: a phase 1 feasibility trial. Nat Med. 2023 Oct;29(10):2481-2488. Epub 2023 Sep 7 PubMed.
Further Reading
News
- In Mice, TREM2 Antibody Mobilizes Microglia, Yet Worsens Tangles
- Potent TREM2 Antibody Stirs Microglia to Prune Plaques in Mice
- TREM2 Latches Onto TDP-43, Spurs Neuroprotective Microglial Mop-Up?
- In Mice, Activating TREM2 Tempers Plaque Toxicity, not Load
- Boost or Block TREM2? Either Way, Therapy May Need Careful Timing
- With TREM2, Timing Is Everything
- Without TREM2, Microglia Run Out of Gas
- In Alzheimer’s, More TREM2 Is Good for You
- TREM2: Diehard Microglial Supporter, Consequences Be DAMed
Primary Papers
- Rachmian N, Medina S, Cherqui U, Akiva H, Deitch D, Edilbi D, Croese T, Salame TM, Ramos JM, Cahalon L, Krizhanovsky V, Schwartz M. Identification of senescent, TREM2-expressing microglia in aging and Alzheimer's disease model mouse brain. Nat Neurosci. 2024 Jun;27(6):1116-1124. Epub 2024 Apr 18 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.