Transient Filament Yields AD and CTE Tau Fibrils
Quick Links
Akin to erratic teenagers, tau may try dozens of amyloid filament styles before settling into the stable configurations found in the brains of people with AD or other tauopathies. This is according to a study published November 29 in Nature, in which scientists led by Sjors Scheres and Michel Goedert of the MRC Laboratory of Molecular Biology, Cambridge, England, used cryo-electron microscopy to spy on tau’s filamentous phases in vitro. The researchers identified an early structure that served as a common precursor for the paired, C-shaped protofilaments found in people with AD, and the slightly more open C configuration found in those with CTE. At the core of these nascent filaments lay the infamous VQIVYK motif, crucial for tau filament formation. Over several hours, C-terminal portions of tau started to form β-sheets, ultimately twisting into J-shaped protofilaments and then the mature, C-shaped pairs.
- On the way from monomers to fibrils, tau twists into dozens of fibrillar conformations.
- CryoEM pegs a common one as precursor for AD and CTE fibrils, at least in vitro.
- This precursor morphs into paired J- and then paired C-shaped protofilaments.
“Altogether, this is a terrific addition to our understanding of how the filaments that define neurodegenerative disease form,” wrote Andrew Stern of Brigham and Women’s Hospital in Boston (comment below). “Although difficult, I hope new techniques can find these intermediates in human tissue. If they’re there, and they last long enough, then they will likely be easier to disassemble than the mature filaments seen so far."
“Reading this paper is like opening a box of chocolates for the holidays and finding a delightful assortment of shapes and structures: treats as food for thought,” wrote Charles Glabe of the University of California, Irvine (comment below).
To Scheres, the findings demonstrate that amyloid formation is much more complicated than previously imagined. The authors suggest that intermediate tau filaments, as opposed to oligomers, beget the fibrils found in AD and CTE.
Over the past decade, studies have converged on the idea that tau, and other amyloidogenic proteins, including Aβ, can tangle into myriad configurations, from soluble oligomeric forms to different fibrillar strains, each imbued with distinct properties (eg., Lasagna-Reeves et al., 2012; May 2014 news; Aug 2017 conference news). For their part, Scheres and Goedert have wielded cryo-EM to zoom in on the different poses tau strikes across neurodegenerative diseases. Their studies have revealed that different tauopathies are marked by distinct tau protofilament folds (Oct 2021 news).
While tau folds into a pair of double-layered C-shaped protofilaments in AD and CTE, other tauopathies are characterized by three- or four-layer folds with entirely different configurations. Yet, the forces that dictate these disease-specific folds remain a mystery. To investigate, the researchers tinkered with in vitro conditions to see if they could coax tau into these different folds in vitro. Previously, they reported that a touch of magnesium in the aggregation reaction leads to AD filaments, while sodium favors the more open Cs of CTE filaments (Lövestam et al., 2022).
For their new study, first author Sofia Lövestam and colleagues used these reaction conditions to monitor metamorphosis from monomers into each of these disease-associated filaments. As their starting material, they used monomers of recombinant tau297-391, which includes the microtubule binding domains known to build the protofilament cores. Even in this stage, nuclear magnetic resonance imaging indicated that certain segments—305-317 in particular—appeared more rigid than others. Scheres likened the monomers to spaghetti, with some segments more al dente than others.
After adding the corresponding salts to initiate fibrillization, Lövestam periodically monitored tau’s transformation via thioflavin-T incorporation and cryo-EM. The first signs of bona fide filaments appeared after 120 minutes. While not detectable with thioflavin-T, these filaments were resolvable via cryo-EM. In the presence of either magnesium or sodium chloride, 100 percent of the resolvable filaments formed a left-handed twist, and their ordered cores comprised none other than the al dente section—residues 302-316—arranged side by side, head-to-toe fashion, with a hydrophobic interface (image below).

Fleeting Filament. The first intermediate amyloid structure emerged after two hours. The core of the left-handed filament comprised tau pairs lying head to toe. [Courtesy of Lövestam et al., 2023.]
Despite its early rise to fibrillar prominence, this so-called first intermediate amyloid, aka FIA, didn’t last long. At 160 minutes, the researchers could no longer detect it. Instead, a dazzling array of other structures started to take shape. In the magnesium-fueled reaction, two dozen filament structures appeared and disappeared over 12 hours, as did many more transient structures that were not clearly resolvable by cryo-EM. The FIA was succeeded by filaments with various J-shaped protofilament cores, in which residues 365-380 doubled over to pair up with the FIA fragment. At later time points, the Js were supplanted by C-shaped protofilaments, such that by 12 hours, most of the filaments resembled the PHFs found in AD brain samples (image below).
In the sodium-fueled CTE reactions, the researchers detected even more folds, resolving 40 filament structures between the FIA and the final, open C-shaped configuration that matches the one found in people with CTE.
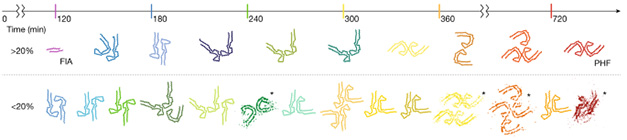
Fun with Folds. Tau assembled into different folds over the course of 12 hours, progressing from the FIA to J-shaped to C-shaped folds, ultimately settling on the AD tau PHF. Structures comprising greater than 20 percent of filaments are shown on top, while scarcer structures are on the bottom. [Courtesy of Lövestam et al., Nature, 2023.]
The fact that tau ultimately folded into fibrillar conformations found in people with AD or CTE supports the use of this in vitro reaction as a model for the earliest events in tau aggregation in the human brain, wrote Stern. However, he, and the authors themselves, pointed out that the intermediate structures are formed from truncated, unphosphorylated tau molecules in pure solution at high concentration with no cofactors other than salts, and therefore their trajectory of aggregation may differ from what happens in the brain. Scheres reiterated that so far, none of the intermediate structures have been found in human brain samples.
Despite these caveats, the study offers a model for how filaments might form. “Unstable but low-barrier intermediates like the FIA can seed the formation of different but stabler conformations, which seed yet stabler conformations,” wrote Stern. “Thus, even the earliest observable intermediates in filament formation are still fibrillar.”
The findings resonate with Stern’s studies with Aβ peptides, in which small, yet fibrillar species, as opposed to oligomers, were found in fluid from the AD brain (Nov 2022 news; May 2023 news). Stern wondered whether similarly small species of tau fibrils—perhaps recoverable at higher speeds of centrifugation—might give rise to the FIA.
The findings also leave open the possibility that a non-fibrillar, oligomeric species of tau is what gives rise to FIA, Scheres acknowledged.
If FIA proves to give rise to tau fibrils in AD and CTE, might targeting this early filament nip tauopathy in the bud? Martin Citron of UCB Pharma in Brussels said that while the findings are interesting from a mechanistic perspective, so far it is unclear what they imply about tau-targeted therapeutic strategies. “However, in the longer run this work could stimulate technically challenging efforts to specifically target such aggregation intermediates with therapeutic antibodies or small molecules,” Citron wrote (comment below).—Jessica Shugart
References
News Citations
- Like Prions, Tau Strains Are True to Form
- Monomeric Seeds and Oligomeric Clouds—Proteopathy News from AAIC
- Flock of New Folds Fills in Tauopathy Family Tree
- Short Aβ Fibrils Easily Isolated from Alzheimer's Brain Fluid
- Paper Alert: Those Fibrils Floating in Brain Fluid Are Toxic
Paper Citations
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Kiritoshi T, Neugebauer V, Jackson GR, Kayed R. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci Rep. 2012;2:700. PubMed.
- Lövestam S, Koh FA, van Knippenberg B, Kotecha A, Murzin AG, Goedert M, Scheres SH. Assembly of recombinant tau into filaments identical to those of Alzheimer's disease and chronic traumatic encephalopathy. Elife. 2022 Mar 4;11 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Lövestam S, Li D, Wagstaff JL, Kotecha A, Kimanius D, McLaughlin SH, Murzin AG, Freund SM, Goedert M, Scheres SH. Disease-specific tau filaments assemble via polymorphic intermediates. Nature. 2024 Jan;625(7993):119-125. Epub 2023 Nov 29 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Brigham and Women's Hospital, Harvard Medical School
Another advance from the Scheres/Goedert collaboration and in vitro filament wizard Sofia Lövestam. The first intermediate filaments (FIAs) seen have a short-ordered core, with presumably the lowest activation energy barrier. Soon thereafter, stabler conformations emerge, first resembling, then exactly copying, the conformation found in Alzheimer’s disease or chronic traumatic encephalopathy. This last fact makes the intermediate observations a model for the earliest events in tau aggregation in the human brain. However, it is important to remember that the intermediate structures here are formed from truncated, unphosphorylated tau molecules in pure solution at high concentration with no cofactors other than salts. The events leading to the first emergence of tau filaments in the human brain may differ from those described here, even if the final structure of the core is the same.
Despite these unavoidable limitations, Lövestam et al. offer a model for how filaments might form: that unstable but low-barrier intermediates like the FIA can seed the formation of different but stabler conformations, which seed yet stabler conformations, etc. Thus, even the earliest observable intermediates in filament formation are still fibrillar. However, I still think their results leave room for non-fibrillar aggregates in their reactions: already at 120 minutes when FIAs are first observed, some are longer than 300 nm, comprising hundreds of stacked monomers. How did these get so big, so fast? Did these long FIAs simply elongate quickly from shorter FIAs formed at 110 minutes or 115 minutes? I do favor this interpretation because the emergence of insolubility (pelleting at 400,000 g for 15 minutes) did not precede the observation of the FIAs. But if the first observable aggregates were so large, then presumably smaller aggregates formed first, and we don’t know their structure. Perhaps centrifuging even faster could isolate still smaller aggregates at earlier timepoints.
Altogether, this is a terrific addition to our understanding of how the filaments that define neurodegenerative disease form. Although difficult, I hope new techniques can find these intermediates in human tissue. If they’re there, and they last long enough, then they will likely be easier to disassemble than the mature filaments seen so far.
University of California, Irvine
Reading this paper from the laboratories of Michel Goedert and Sjors Scheres at the MRC in Cambridge, U.K., is like opening a box of chocolates for the holidays and finding a delightful assortment of shapes and structures: treats as food for thought. I have only begun to appreciate this, but some of my initial sensory impressions include the following: The pathways of aggregation and the plethora of intermediate structures (perhaps not a “cloud” of amyloid structures, but more than a few drops) is not likely to be unique to tau, but rather more likely to be common to other amyloids that have been shown to have a “curvilinear,” “protofibril” intermediate by negative stain electron microscopy or atomic force microscopy. This would include other disease-associated amyloids, such as Aβ, α-synuclein, TDP43, and perhaps all amyloid that forms parallel, main-chain, intermolecular hydrogen-bonded β-sheet fibrils.
The data clearly illuminate the pathways for fibril formation and identify a series of intermediate structures. It also points to the need to have better tools, such as fluorescent dyes and monoclonal antibodies that can distinguish these different structures, to facilitate the study of these polymorphic structures in vitro and in vivo. For antibodies, this also illustrates that merely knowing the location of the epitope is insufficient for defining the binding site because the same sequence can exist in multiple different structures with different solvent accessibilities. This may also be crucial for finding the most effective therapeutic monoclonal antibodies or in the case where several different polymorphs contribute to pathogenesis, a polyclonal immune response may be more effective.
Does the term “oligomer” have any significant meaning for this class of amyloid structures? Since the structural variation is due to variations in the location and stacking of the β-sheets, the structure of individual polymorphs is the same over a broad range of lengths that can vary by a single polypeptide chain. This seems like arguing over how large a slice must be before it is called a sausage.
Lastly there is the question of which comes first, the first intermediate amyloid (FIA/protofibril) or the prefibrillar oligomer (PFO), a term coined by Sir Christopher Dobson to describe another class of intermediate structures? Based on the binding of PFO-specific monoclonal antibodies that also recognize known antiparallel β structures such as β cylindrins and membrane pores formed by bacterial toxins, PFOs, and seemingly related structures such as annular protofibrils, appear to be intermolecularly hydrogen-bonded aggregates containing antiparallel β-sheets. As such, there does not seem to be any structural relationship among pathways, and therefore no prediction of kinetic priority.
UCB Pharma Belgium
This is an interesting, in-vitro study with recombinant tau (297–391), analyzing structures of intermediate amyloid species that form during the assembly into filaments containing the Alzheimer or CTE fold, leading to a model in which prefibrillar, oligomeric species are not required.
While very interesting from a mechanistic perspective, I do not see the immediate therapeutic implication, as the tau-directed therapies currently in development do not specifically address the intermediates, or depend on a specific hypothesis about such intermediates. However, in the longer run, this work could stimulate—technically challenging—efforts to specifically target such aggregation intermediates with therapeutic antibodies or small molecules.
University of Texas, Southwestern Medical Center
UT Southwestern Medical Center
This work represents another tour de force from the Goedert and Scheres groups. They have allowed a tau fragment to spontaneously fibrillize under different conditions, which lead either to a conformation that mimics that found in Alzheimer’s disease, or, using a different salt, a conformation found in chronic traumatic encephalopathy. By stopping the reaction at distinct time points and resolving dozens of emergent assemblies, they have revealed oligomeric protofibril intermediates that precede the final, dominant, disease-specific conformation. The studies suggest that the aggregation of the tau fragment is “pluripotential” in terms of resultant structures, and that over time, in a test tube with the right conditions, these can be driven to “collapse” toward a dominant configuration. It remains to be seen, of course, whether this process occurs in cells, and whether the same rules will apply to full-length tau.
We were excited to see these results, because they confirm and extend findings of the last several decades from multiple labs supporting the central role of the amyloidogenic motif—VQIVYK—in tau assembly, especially work by Mandelkow, Zweckstetter, Eisenberg, and ourselves (von Bergen et al., 2000; Mukrasch et al., 2005; Sawaya et al., 2007). This motif is also essential for stabilization of disease-specific fibrils, as described by our and other labs (Mullapudi et al., 2023; van der Kant et al., 2022).
In many ways, this work supports, and is predicted by, our hypothesis that tau monomer exists in two general, and relatively stable, conformational ensembles. Based on isolation and characterization of the earliest, smallest, detectable forms of tau seeds in vivo, we concluded that local conformational change in tau surrounding the DNIKHVPGGGVQIVYK motif underlies conversion of monomer from an inert (Mi) to a seed-competent form (Ms). Ms spontaneously self-assembles, serves as a template for further fibril growth, and is biochemically purifiable from AD brain and from recombinant protein (Mirbaha, 2018; Chen et al., 2019; Hou et al., 2021). It is also the earliest detectable seed-competent form of tau, appearing in a mouse model long before larger assemblies (Mirbaha, et al., 2022).
The aggregation potential of Ms appears to be based on exposure of amyloidogenic sequences that are normally masked by upstream amino acids. Most recently, monoclonal antibodies raised against this epitope, designed to mimic the critical exposed tau sequence in Ms, were remarkably efficient at discriminating seed-competent tau from the vast majority of inert tau monomer (Hitt, 2023). We hope these reagents will be useful to isolate these early seed competent forms from brain for imaging studies.
We are optimistic that advances, such as these reported by Lovestam et al., in combination with other efforts around the world, will soon deliver much more effective diagnosis and therapy.
References:
von Bergen M, Friedhoff P, Biernat J, Heberle J, Mandelkow EM, Mandelkow E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc Natl Acad Sci U S A. 2000 May 9;97(10):5129-34. PubMed.
Mukrasch MD, Biernat J, von Bergen M, Griesinger C, Mandelkow E, Zweckstetter M. Sites of tau important for aggregation populate {beta}-structure and bind to microtubules and polyanions. J Biol Chem. 2005 Jul 1;280(26):24978-86. PubMed.
Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, Apostol MI, Thompson MJ, Balbirnie M, Wiltzius JJ, McFarlane HT, Madsen AØ, Riekel C, Eisenberg D. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature. 2007 May 24;447(7143):453-7. PubMed.
Mullapudi V, Vaquer-Alicea J, Bommareddy V, Vega AR, Ryder BD, White CL 3rd, Diamond MI, Joachimiak LA. Network of hotspot interactions cluster tau amyloid folds. Nat Commun. 2023 Feb 16;14(1):895. PubMed.
van der Kant R, Louros N, Schymkowitz J, Rousseau F. Thermodynamic analysis of amyloid fibril structures reveals a common framework for stability in amyloid polymorphs. Structure. 2022 Aug 4;30(8):1178-1189.e3. Epub 2022 May 23 PubMed.
Mirbaha H, Chen D, Morazova OA, Ruff KM, Sharma AM, Liu X, Goodarzi M, Pappu RV, Colby DW, Mirzaei H, Joachimiak LA, Diamond MI. Inert and seed-competent tau monomers suggest structural origins of aggregation. Elife. 2018 Jul 10;7 PubMed.
Chen D, Drombosky KW, Hou Z, Sari L, Kashmer OM, Ryder BD, Perez VA, Woodard DR, Lin MM, Diamond MI, Joachimiak LA. Tau local structure shields an amyloid-forming motif and controls aggregation propensity. Nat Commun. 2019 Jun 7;10(1):2493. PubMed.
Hou Z, Chen D, Ryder BD, Joachimiak LA. Biophysical properties of a tau seed. Sci Rep. 2021 Jun 30;11(1):13602. PubMed.
Mirbaha H, Chen D, Mullapudi V, Terpack SJ, White CL 3rd, Joachimiak LA, Diamond MI. Seed-competent tau monomer initiates pathology in a tauopathy mouse model. J Biol Chem. 2022 Aug;298(8):102163. Epub 2022 Jun 22 PubMed.
Hitt BD, Gupta A, Singh R, Yang T, Beaver JD, Shang P, White CL 3rd, Joachimiak LA, Diamond MI. Anti-tau antibodies targeting a conformation-dependent epitope selectively bind seeds. J Biol Chem. 2023 Nov;299(11):105252. Epub 2023 Sep 14 PubMed.
Make a Comment
To make a comment you must login or register.