In Parkinson’s, Synuclein Fibrils Fold Differently Than in MSA
Quick Links
There’s a new twist to the Lewy body story. In a manuscript uploaded July 12 to bioRXiv, scientists led by Sjors Scheres and Michel Goedert at the Medical Research Council Laboratory of Molecular Biology, Cambridge, England, present the cryo-EM structure of α-synuclein fibrils isolated from people who had died with Parkinson’s, Parkinson’s dementia, or dementia with Lewy bodies. The surprising upshot: Fibrils from all three diseases grow from one identical protofilament.
- Cryo-EM structures of α-synuclein from PD, PDD, and DLB are identical
- Their “Lewy fold” barely resembles protofilaments from MSA.
- The PD/PDD/DLB protofilament does resemble recombinant α-synuclein structures.
This stands in stark contrast to the fibrils in people who had multiple system atrophy, another α-synucleinopathy. Previously, Goedert and colleagues had identified two types of α-synuclein fibrils in MSA, each comprising two protofilaments for a total of four unique folds. The PD protofilaments adopt yet another structure. The findings suggest that, as with tau, the conformations of α-synuclein filaments depend on the neurodegenerative disease.
First author Yang Yang and colleagues extracted α-synuclein filaments postmortem from the cingulate cortices of one person with PD, one with DLB, and two people with PDD, plus filaments from the frontal cortices of two other people who had DLB. High-resolution cryo-EM showed that the fibrils were all the same, comprising a single protofilament core (image below).
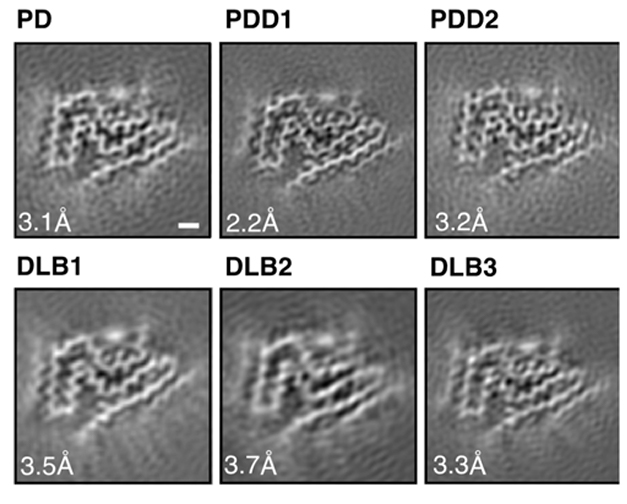
Three Diseases—One Structure. Synuclein protofilaments in PD, PDD, and DLB all adopt the same fold. [Courtesy of Yang et al., bioRXiv 2022.]
The researchers dubbed this core the “Lewy fold.” It comprises amino acids 31-100 of the protein in a three-layer structure consisting of nine β-sheets. The first eight sheets are corrugated into two layers, the ninth makes up the third layer by itself (image below).
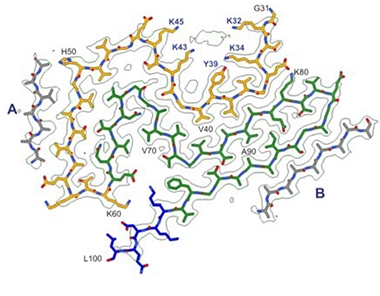
Parkinson’s Protofibril. The core of the PD protofibril includes amino acids 31-60 of the N-terminal domain (orange), all of the hydrophobic region (AAs 61-95, green), and five amino acids of the C-terminal domain (blue). [Courtesy of Yang et al., bioRXiv 2022.]
Two other peptides lay adjacent to β-sheets 5 and 9 (image below). Yang was unable to identify these “islands” because they lacked distinct side-chain densities in the cryo-EM. In addition, an unidentified non-peptide entity fits into a groove between β-sheets 1 to 3. Similarly mysterious entities have been found in protofilaments of α-synuclein in MSA and in tau filaments isolated from people who had chronic traumatic encephalopathy and some primary tauopathies (May 2020 news; Mar 2019 news; Oct 2021 news). The authors believe this unknown molecule might be an important co-factor for the formation of the Lewy fold.
At first blush, the differences between the Lewy fold and the MSA folds seem stark (image below). However, a small section of α-synuclein—amino acids 61-72—adopts the same conformation in both.
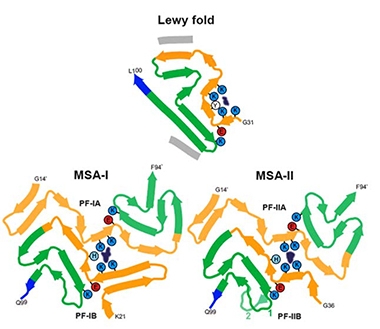
Lewy Fold. Their nine β-sheets (ribbons) set the α-synuclein protofilament in PD, PDD, and DLB apart from the four found in MSA, which adopt different folds (bottom). In the Lewy fold, additional, unidentified peptides (gray) stack against β-sheets 5 and 9, while a non-peptide factor (gray blotch) wedges into a groove formed by sheets 1-3. It is surrounded by four lysines (blue circles) and a tyrosine (white). [Courtesy of Yang et al., bioRXiv 2022.]
Intriguingly, the Lewy fold resembles some of the structures adopted by recombinant α-synuclein in vitro. When the MSA structures were reported, they caused a bit of a letdown for the field, since they bore little resemblance to the structures of recombinant synucleins that were being tested in various experimental paradigms and as templates for PET ligands (see Mar 2020 conference news). The Lewy fold suggests that at least some of those recombinant structures may be physiologically relevant, after all.
Amino acids 32-41 and 70-82 overlay with an in vitro protofibril phosphorylated at tyrosine 39, though Yang found no evidence that this residue was phosphorylated in the Lewy fold, as had been previously reported (Zhao et al., 2020). Likewise, amino acids 69-98 overlay with the same residues in filaments of an N-terminally truncated recombinant protofibril (McGlinchey et al., 2021). The atomic density of the peptide island that cozies up to β-sheet 9 overlays with amino acids 14-23 in that same recombinant structure, while the interface between β-sheet 5 and island B resembles the interface between a different α-synuclein protofilament dimer formed in vitro (Guerrero-Ferreira et al., 2019).
Indeed, Scheres and Goedert told Alzforum that the most important thing to do now would be to find ways to create the Lewy and MSA folds in vitro, as they recently did for tau filaments from AD and CTE (Lövestam et al., 2022). “Being able to make these in the lab would open up new avenues to do functional studies with these filaments,” wrote Scheres. They would also serve as templates for developing PET ligands, he said.
What about other α-synuclein folds? That PD, PDD, and DLB folds are identical supports the idea that these diseases are closely related. Clinically, as well, MSA differs more from PD, PDD, DLB than these three do to each other.
The how about α-synuclein deposits seen in Alzheimer's? Would they be the same? Scheres said they are not currently planning to study those. “Perhaps filaments from the brain versus those from the periphery would be the most interesting to study,” wrote Goedert. There is evidence that synucleinopathies begin with aggregation of the protein in the gastrointestinal tract (see 2011 news series), and α-synuclein deposits have been documented in skin, as well (Oct 2020 news). Folds formed by synuclein mutations would also be interesting to study, Goedert said.—Tom Fagan
References
News Citations
- Paper Alert: CryoEM Structures of α-Synuclein Published
- Traumatic Tau: Filaments from CTE Share Distinct Structure
- Flock of New Folds Fills in Tauopathy Family Tree
- Behold the First Human α-Synuclein CryoEM Fibril Structure
- No Skin Off Your Nose: New Way to Diagnosing Parkinson’s?
Series Citations
Paper Citations
- Zhao K, Lim YJ, Liu Z, Long H, Sun Y, Hu JJ, Zhao C, Tao Y, Zhang X, Li D, Li YM, Liu C. Parkinson's disease-related phosphorylation at Tyr39 rearranges α-synuclein amyloid fibril structure revealed by cryo-EM. Proc Natl Acad Sci U S A. 2020 Aug 18;117(33):20305-20315. Epub 2020 Jul 31 PubMed.
- McGlinchey RP, Ni X, Shadish JA, Jiang J, Lee JC. The N terminus of α-synuclein dictates fibril formation. Proc Natl Acad Sci U S A. 2021 Aug 31;118(35) PubMed.
- Guerrero-Ferreira R, Taylor NM, Arteni AA, Kumari P, Mona D, Ringler P, Britschgi M, Lauer ME, Makky A, Verasdonck J, Riek R, Melki R, Meier BH, Böckmann A, Bousset L, Stahlberg H. Two new polymorphic structures of human full-length alpha-synuclein fibrils solved by cryo-electron microscopy. Elife. 2019 Dec 9;8 PubMed.
- Lövestam S, Koh FA, van Knippenberg B, Kotecha A, Murzin AG, Goedert M, Scheres SH. Assembly of recombinant tau into filaments identical to those of Alzheimer's disease and chronic traumatic encephalopathy. Elife. 2022 Mar 4;11 PubMed.
Further Reading
Primary Papers
- Yang Y, Shi Y, Schweighauser M, Zhang X, Kotecha A, Murzin AG, Garringer HJ, Cullinane PW, Saito Y, Foroud T, Warner TT, Hasegawa K, Vidal R, Murayama S, Revesz T, Ghetti B, Hasegawa M, Lashley T, Scheres SH, Goedert M. Structures of α-synuclein filaments from human brains with Lewy pathology. Nature. 2022 Oct;610(7933):791-795. Epub 2022 Sep 15 PubMed. bioRxiv. PubMed
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.