Wolframin-1 Cells: Tau’s Launch Pad from Entorhinal Cortex to Hippocampus?
Quick Links
The spread of tau pathology from the entorhinal cortex into the neighboring hippocampus region of the brain is thought to herald the onset of clinical symptoms in AD, but exactly what path does tau take from one region to the next? Both, after all, boast complex microanatomies comprising distinct cell types and circuits. In the September 15 Science Translational Medicine, scientists led by Tsuneya Ikezu, Mayo Clinic in Jacksonville, Florida, peg a subtype of pyramidal neuron in the entorhinal cortex as the starting point.
- In people with early AD, Wolframin-1 neurons in the EC harbor tau pathology.
- Wolframin-1 neurons in the EC connect directly to CA1 neurons in the hippocampus.
- In mice, misfolded tau expressed in the former travels to the latter.
- When expressed in mouse Wolframin-1 neurons, pathologic tau dampens hippocampal synapses and memory.
Brandishing a protein called wolframin-1 (Wfs1), these cells project their axons directly toward CA1 neurons in the hippocampus. First author Jean-Christophe Delpech and colleagues reported that misfolded tau rides this circuit into the hippocampus, where it promptly douses the activity of local neurons and hobbles associative memory in mice.
The researchers spotted corresponding Wfs1+ neurons in humans. In people who had died while at early stages of AD, these cells specifically were prone to harbor tau tangles, suggesting that misfolded tau could embark from Wfs1+ neurons into the hippocampus and beyond.
“This study beautifully shows in a mouse model that the direct circuit from a previously identified subset of the entorhinal cortex layer II neurons to the hippocampal CA1 field propagates the abnormally phosphorylated tau protein, which likely contributes to a memory impairment in the early stage of Alzheimer's disease,” commented Susumu Tonegawa of Massachusetts Institute of Technology in Boston. Tonegawa had previously identified this circuit.
In the earliest stages of AD, tau tangles spread from layer II of the EC (ECII) into the CA1 region of the hippocampus. Many researchers believe that tau moves from one region to another via trans-synaptic propagation, but defining how this happens between ECII and the hippocampus has been challenging. In mouse models expressing human tau in ECII, tau predominantly moves from ECII to the dentate gyrus of the hippocampus via a circuit called the perforant pathway (Feb 2012 news; de Calignon et al., 2012). Trouble is, in people with Alzheimer's, the dentate gyrus remains impervious to tau tangles until later stages of disease, despite its adjacent proximity to CA1.
Why doesn't tau travel from ECII into the CA1 field of the hippocampus in mice as it does in humans? Ikezu and colleagues reasoned that perhaps previous models were starting off with the wrong type of neuron to send tau on its way. In rodents and in humans, two neuronal types predominate within layer II of the entorhinal cortex: multipolar stellate neurons, and pyramidal neurons (Naumann et al., 2016; Witter et al., 2017). While the stellate neurons project to the dentate gyrus as part of the perforant pathway, a subset of the pyramidal cells project to CA1 of the hippocampus. They form a circuit known as the temporoammonic pathway, which was discovered by Tonegawa in 2014. These pyramidal “island cells” form small clusters within a sea of stellate neurons in ECII, and they express Wfs1 (Kitamura et al., 2014).
Might Wfs1+ neurons dispatch tau to the hippocampus? This is the question Delpech and colleagues went after. They acquired Wfs1-Cre mice from Tonegawa, and injected an adeno-associated virus expressing a Cre-inducible version of human tau bearing the P301L mutation directly into their ECII. It worked: Only Wfs1+ neurons expressed the mutated human tau. A month later, the scientists spotted phosphorylated human tau accumulating in the CA1 region of the hippocampus, but not in the dentate gyrus. This pathway therefore more closely modeled tau’s trajectory in humans, the scientists believe.
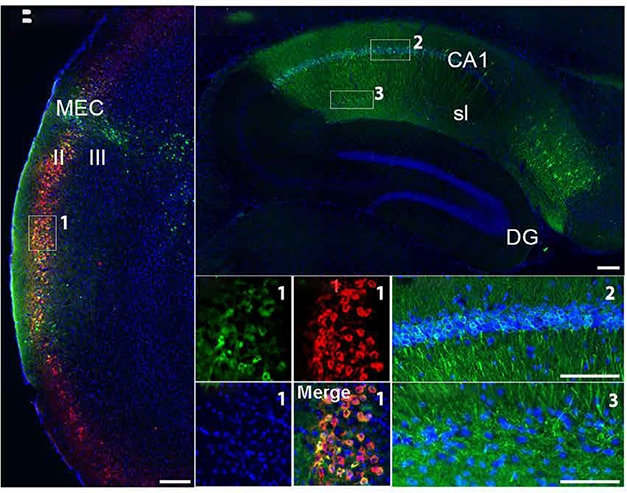
Wolframin Express. In Wfs1-Cre mice (left), only Wolframin neurons in ECII (red) express human P301L tau (green). One month later, human tau appears in CA1, but not dentate gyrus, of the hippocampus (right). [Courtesy of Delpech et al., Science Translational Medicine, 2021.]
Using AAVs expressing fluorescent proteins to mark Wfs1+ neurons in the EC and hippocampal CA1 neurons, the researchers established that Wfs1+ neurons projected axons directly from ECII to dendrites of CA1 neurons. Immunoelectron microscopy confirmed this direct synaptic connection, also revealing that some Wfs1+ ECII neurons connected to inhibitory interneurons in the hippocampus.
Does the spread of misfolded tau influence synaptic signaling between EC Wfs1+ neurons and hippocampal CA1 neurons? The researchers brought a three-prong approach to this question, using optogenetic, chemogenic, and electrophysiological methods to test how tau affected the road on which it traveled. In a nutshell, they found that P301L tau expression in Wfs1+ EC neurons not only rendered those cells less likely to fire, but also dampened the CA1 neuron's responsiveness. Essentially, misfolded tau tamped down both pre- and post-synaptic signals in the circuit.
In keeping with the known role of the temporoammonic circuit in associative memory, the researchers also found that mice expressing P301L-tau in Wfs1+ neurons were less able to recognize the context in which they had previously received a foot shock.
Do such Wfs1+ cells exist in the human EC, and if so, could they launch tau early in AD? In postmortem brain samples from 12 people who had died at different stages of AD, the scientists detected Wfs1+ pyramidal neurons in ECII of all samples. CP13 and PHF1 antibodies against misfolded, phosphorylated tau labeled a subset of Wfs1+ neurons as being burdened with tau pathology. The percentage of tauopathy-bearing Wfs1+ cells was higher in people at early disease stages—those with CDR scores of 0.5 or 2—and lower in cognitively normal people or those in late stages, i.e., CDR 0 or 3.

Tau: Early Riser. In postmortem brain samples from people who died at different stages of disease (based on CDR scores), the proportion of Wolframin-1 neurons in ECII with tau pathology rose early in AD. [Courtesy of Delpech et al., Science Translational Medicine, 2021.]
Such cross-sectional data can only hint at tau’s trajectory throughout the course of AD. Still, Ikezu said they support the idea that these cells set tauopathy in motion.
To study further how tau propagates along the temporoammonic circuit, the Ikezu lab is stereotactically injecting tiny amounts of tau extracted from AD brain. He thinks therapeutics aimed at nipping the tauopathy phase of AD in the bud could be tested in this model.
Wolframin 1 itself might warrant a look as a target. Expressed in the endoplasmic reticulum and mitochondria, Wfs1 is involved in the cellular stress response. Some studies suggest that neurons expressing it are more prone to accumulate tau, though, paradoxically, deficiency in Wfs1 exacerbates tau pathology (Chen et al., 2020; Li et al., 2020). Recessive mutations in Wfs1 cause Wolfram syndrome, a metabolic, neurodegenerative disease marked by juvenile diabetes and loss of sight and hearing (Barrett et al., 1995).—Jessica Shugart
References
News Citations
Paper Citations
- de Calignon A, Polydoro M, Suárez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012 Feb 23;73(4):685-97. PubMed.
- Naumann RK, Ray S, Prokop S, Las L, Heppner FL, Brecht M. Conserved size and periodicity of pyramidal patches in layer 2 of medial/caudal entorhinal cortex. J Comp Neurol. 2016 Mar 1;524(4):783-806. Epub 2015 Sep 9 PubMed.
- Witter MP, Doan TP, Jacobsen B, Nilssen ES, Ohara S. Architecture of the Entorhinal Cortex A Review of Entorhinal Anatomy in Rodents with Some Comparative Notes. Front Syst Neurosci. 2017;11:46. Epub 2017 Jun 28 PubMed.
- Kitamura T, Pignatelli M, Suh J, Kohara K, Yoshiki A, Abe K, Tonegawa S. Island cells control temporal association memory. Science. 2014 Feb 21;343(6173):896-901. Epub 2014 Jan 23 PubMed.
- Chen S, Venkaraman L, Liang J, Nakano Y, Hernandez Villegas NE, Brown C, Urano F, Koks S, Serrano GE, Beach TG, Davies P, Diamond M, Duff KE, Fu H. Deficiency of WFS1 increases vulnerability to pathological tau in vitro and in vivo. Alzheimer's & Dementia, December 2020
- Li L, Venkataraman L, Chen S, Fu H. Function of WFS1 and WFS2 in the Central Nervous System: Implications for Wolfram Syndrome and Alzheimer's disease. Neurosci Biobehav Rev. 2020 Nov;118:775-783. Epub 2020 Sep 17 PubMed.
- Barrett TG, Bundey SE, Macleod AF. Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet. 1995 Dec 2;346(8988):1458-63. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Delpech JC, Pathak D, Varghese M, Kalavai SV, Hays EC, Hof PR, Johnson WE, Ikezu S, Medalla M, Luebke JI, Ikezu T. Wolframin-1-expressing neurons in the entorhinal cortex propagate tau to CA1 neurons and impair hippocampal memory in mice. Sci Transl Med. 2021 Sep 15;13(611):eabe8455. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.