Microglia Instigate ‘Chemofog’ by Squelching Myelination
Quick Links
We can now add myelination to the list of critical functions put on hold by cantankerous microglia. A paper published May 10 in Neuron reported that in mice treated with the widely used chemotherapy drug methotrexate, microglia became activated and, like so many wet blankets, doused neuronal expression of brain-derived growth factor (BDNF). This stifled the development of oligodendrocytes, thwarting myelination. As a consequence, the mice suffered memory loss, modeling the phenomenon known as “chemo-fog” in cancer patients. Led by Michelle Monje at Stanford University, the researchers restored myelination and memory by boosting BDNF signaling in oligodendrocytes with a small-molecule drug. They propose that tempering microglial activation could have a similar effect.
- Methotrexate activates microglia, leading neurons to reduce BDNF expression.
- Low BDNF reduces activity-dependent myelination by oligodendrocytes, and triggers memory loss.
- The small molecule LM22A-4 restores myelination and cognition.
The study casts reactive microglia as blockers of myelination, and raises the possibility that these cells could cause similar problems in other contexts in which they are aberrantly activated, such as in neurodegenerative disease, Monje noted.
Oligodendrocytes form the fatty myelin sheath that insulates axons in the brain and enables rapid neuronal signaling. Although axons become myelinated by default during development, in recent years, researchers have discovered that neuronal activity can also trigger myelination throughout life. This activity-dependent myelination is thought to facilitate structural plasticity in the brain (Gibson et al., 2014; Bechler et al., 2015; Mount and Monje, 2017). Earlier this year, Monje’s group reported that in mice treated with methotrexate, activated microglia soured this form of myelination by somehow derailing the development of oligodendrocytes (Gibson et al., 2019).
In their current study, first author Anna Geraghty and colleagues fleshed out the pathway. They used an optogenetic mouse model with an implanted probe to stimulate premotor neurons that project axons into the corpus callosum—the dense white-matter tract that separates the hemispheres of the brain. Stimulating these neurons promoted proliferation of nearby oligodendrocyte progenitor cells (OPCs), and the myelin sheath thickened. Methotrexate blocked this activity-dependent myelination, unless microglia were first depleted with the CSF-1R inhibitor PLX5622. This suggested that microglia somehow facilitate methotrexate’s negative impacts on myelination, but how?
The scientists hypothesized that BDNF might play a role, as a recent study linked a BDNF polymorphism with decreased risk for cognitive impairment after chemotherapy in women (Ng et al., 2016). Indeed, they found that in response to methotrexate treatment, BDNF mRNA and protein plummeted in the deep layers of the mice’s frontal cortices. Depleting microglia prevented this dip in BDNF, suggesting that microglia somehow hush BDNF expression in neurons.
BDNF signals through the TrkB receptor on OPCs, triggering their proliferation and ultimate differentiation into functional oligodendrocytes. Using an intricate combination of mouse models, the researchers pieced together the pathway leading from methotrexate to flagging myelin production. They reported that under healthy conditions, neural activity stimulates BDNF expression, which signals through the TrKB receptor on OPCs, leading to their proliferation and ultimately, a boost in myelination. Methotrexate derails this by activating microglia, which somehow block neurons from producing BDNF. The loss of myelination prevented mice from distinguishing novel from familiar objects, a measure of memory loss.
Finally, the researchers found that they could circumvent the testy microglia and their muted neuronal bystanders by stimulating OPCs directly, using the small molecule TrkB receptor agonist LM22A-4. The treatment not only restored activity-dependent myelination, but also prevented memory loss in response to methotrexate.
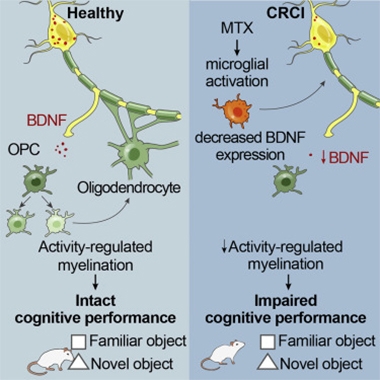
Microglia Make Chemobrain. In the healthy brain (left), neural stimulation promotes the release of BDNF, which triggers OPC proliferation and myelination. In animals treated with methotrexate, activated microglia douse BDNF expression by neurons, slowing myelination and causing memory loss. [Courtesy of Geraghty et al., Neuron, 2019.]
How microglia turn down neuronal BDNF production in response to methotrexate is unclear, Monje said. In response to methotrexate treatment, microglia appear to shift from a more trophic, homeostatic state to an activated, neurotoxic one. Although this general state change has been noted in neurodegenerative disease as well, Monje pointed out that microglia likely undergo distinct changes depending on the disease setting. She proposed that despite these differences, microglia could similarly shut down BDNF expression in the context of other pathologies. The growth factor is thought to play a role in AD, as a polymorphism in the gene hastens cognitive decline in people with Aβ accumulation (Oct 2014 news). Whether flagging activity-dependent myelination mediates this cognitive effect is unclear, although white-matter abnormalities have been associated with AD as well (for review, see Nasrabady et al., 2018).
“This speaks to the notion that no microglia is far better than bad microglia,” noted Kim Green at the University of California, Irvine, who pioneered the use of CSF-1R inhibitors to deplete microglia (Apr 2014 news). His lab reported similar beneficial effects of booting the immune cells following radiotherapy (Acharya et al., 2016). “Overall, these results shed light on the upstream causes of ‘chemo-brain’ and suggest that targeting of microglia would be broadly beneficial,” Green wrote, cautioning that more research is needed before attempting to do so in humans. “How glial cells can regulate neuronal form and function may be integral to the pathogenesis of diseases such as AD,” he added.
However, Tjakko Van Ham of Erasmus University in Rotterdam noted that although microglial depletion is beneficial in certain settings, other data paint a picture of microglia as supporting myelin (for review, see Miron, 2017). He co-authored a case study of an infant with a homozygous mutation in CSF-1R, who was born with profound white-matter abnormalities, including a complete lack of the corpus callosum (Apr 2019 news). At autopsy, the infant’s brain was devoid of microglia, suggesting that the cells play a key role in supporting the development of white-matter structures. “Clearly, strong evidence exists that enhancing as well as reducing microglia activity can be beneficial, but importantly, the specific circumstances, i.e. brain region, age of patient, type of disease, and the state of the microglia, could prove critical in determining the success of such approaches,” Van Ham wrote to Alzforum.
Monje told Alzforum that her lab is investigating whether the microglia that repopulate the brain following depletion regain a calm and myelin-friendly state, even in mice previously treated with methotrexate. She proposed the TrkB agonist and microglial depletion as complementary strategies for warding off chemo-fog, and perhaps for other neurological disorders.—Jessica Shugart
References
News Citations
- Do Neurotrophin and ApoE Together Exacerbate Alzheimer's?
- Microglial Magic: Drug Wipes Them Out, New Set Appears
- Life Without Microglia: Rare Cases of CSF-1R Mutations Paint a Grim Picture
Paper Citations
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014 May 2;344(6183):1252304. Epub 2014 Apr 10 PubMed.
- Bechler ME, Byrne L, Ffrench-Constant C. CNS Myelin Sheath Lengths Are an Intrinsic Property of Oligodendrocytes. Curr Biol. 2015 Sep 21;25(18):2411-6. Epub 2015 Aug 27 PubMed.
- Mount CW, Monje M. Wrapped to Adapt: Experience-Dependent Myelination. Neuron. 2017 Aug 16;95(4):743-756. PubMed.
- Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L, Woo PJ, Barres BA, Liddelow S, Vogel H, Monje M. Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell. 2019 Jan 10;176(1-2):43-55.e13. Epub 2018 Dec 6 PubMed.
- Ng T, Teo SM, Yeo HL, Shwe M, Gan YX, Cheung YT, Foo KM, Cham MT, Lee JA, Tan YP, Fan G, Yong WS, Preetha M, Loh WJ, Koo SL, Jain A, Lee GE, Wong M, Dent R, Yap YS, Ng R, Khor CC, Ho HK, Chan A. Brain-derived neurotrophic factor genetic polymorphism (rs6265) is protective against chemotherapy-associated cognitive impairment in patients with early-stage breast cancer. Neuro Oncol. 2016 Feb;18(2):244-51. Epub 2015 Aug 19 PubMed.
- Nasrabady SE, Rizvi B, Goldman JE, Brickman AM. White matter changes in Alzheimer's disease: a focus on myelin and oligodendrocytes. Acta Neuropathol Commun. 2018 Mar 2;6(1):22. PubMed.
- Acharya MM, Green KN, Allen BD, Najafi AR, Syage A, Minasyan H, Le MT, Kawashita T, Giedzinski E, Parihar VK, West BL, Baulch JE, Limoli CL. Elimination of microglia improves cognitive function following cranial irradiation. Sci Rep. 2016 Aug 12;6:31545. PubMed.
- Miron VE. Microglia-driven regulation of oligodendrocyte lineage cells, myelination, and remyelination. J Leukoc Biol. 2017 May;101(5):1103-1108. Epub 2017 Mar 1 PubMed.
Further Reading
Papers
- Spangenberg EE, Lee RJ, Najafi AR, Rice RA, Elmore MR, Blurton-Jones M, West BL, Green KN. Eliminating microglia in Alzheimer's mice prevents neuronal loss without modulating amyloid-β pathology. Brain. 2016 Apr;139(Pt 4):1265-81. Epub 2016 Feb 26 PubMed.
Primary Papers
- Geraghty AC, Gibson EM, Ghanem RA, Greene JJ, Ocampo A, Goldstein AK, Ni L, Yang T, Marton RM, Paşca SP, Greenberg ME, Longo FM, Monje M. Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron. 2019 Jul 17;103(2):250-265.e8. Epub 2019 May 20 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Erasmus University Medical Center
This is a very interesting paper providing further evidence, in a mouse model, for how chemotherapy—specifically methotrexate/MTX—could affect cognition, and how this involves multiple glial cell types, eventually leading to defective adaptive myelination. To induce and measure adaptive myelination, the authors used an elegant optogenetics strategy based on optical channelrhodopsin stimulation in cortical neurons. Only quite recently, the same group identified that MTX negatively affects oligodendrocytes and their precursors, and that a tri-glial mechanism explains some of these negative effects of chemotherapy on the brain (Gibson et al., 2019). The current study also identifies that under methotrexate treatment, microglia appear, at least partly, responsible for this effect on myelination by decreasing neuronal expressed/secreted BDNF.
One question that comes to mind that is not addressed in this paper is how microglia then affect BDNF expression. Perhaps they could phagocytize the secreted vesicles that contain BDNF. The paper adds to a growing list of reported beneficial effects of depletion of microglia under specific disease conditions in mouse models.
There is also quite strong evidence that suggests that there are beneficial effects of microglia on myelination mediated by yet-to-be-identified mechanisms. We recently showed that, in rare human infantile and adult-onset white matter diseases caused by bi-allelic and homozygous or heterozygous mutations in CSF1R, there is an almost complete loss and a reduced density of microglia and regional loss (Oosterhof et al., 2019; Oosterhof et al., 2018). We hypothesize that in these cases, insufficient microglia numbers or activity could lead to defective myelination and/or loss of myelin. This would suggest a more trophic, supportive role of microglia which could be lost, explaining the detrimental effect on the myelin.
Evidence of such a beneficial/positive role of microglia in myelination is provided by several studies, for example one, using a mouse model, that identified that the growth factor IGF1 secreted by microglia is needed for myelination in postnatal brain development (Wlodarczyk et al., 2017). Another study showed a role of microglia in adult myelination, by using microglia depletion by inhibition of CSF1R (Hagemeyer et al., 2017). Both studies provide strong evidence that microglia affect under homeostasis the oligodendrocytes and their progenitor cells (OPCs). Other recent work shows that replenishing microglia in mouse with homozygous Csf1r mutations, which have essentially no microglia and severe brain defects, rescues some of these defects as viability of these mice is strongly increased (Bennett et al., 2018, Neuron).
In human, transplantation of hematopoietic cells in metachromatic leukodystrophy can stabilize disease and increase burden-free survival (van Rappard et al., Blood, 2016). Anecdotal evidence exists that such transplantations also could benefit patients with heterozygous CSF1R mutations, which could possibly occur by enhancing brain macrophage or microglia function.
Clearly, strong evidence exists that enhancing as well as reducing microglia activity can be beneficial, but importantly, the specific circumstances, i.e. brain region, age of patient, type of disease, and the state of the microglia, could prove critical in determining the success of such approaches. That said, Geraghty et al. in the current work appear to neatly bypass these complex issues by directly acting at the downstream consequence, and provide a pharmacological means to enhance adaptive myelination, by acting on the TrkB receptor on OPCs.
References:
Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L, Woo PJ, Barres BA, Liddelow S, Vogel H, Monje M. Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell. 2019 Jan 10;176(1-2):43-55.e13. Epub 2018 Dec 6 PubMed.
Oosterhof N, Chang IJ, Karimiani EG, Kuil LE, Jensen DM, Daza R, Young E, Astle L, van der Linde HC, Shivaram GM, Demmers J, Latimer CS, Keene CD, Loter E, Maroofian R, van Ham TJ, Hevner RF, Bennett JT. Homozygous Mutations in CSF1R Cause a Pediatric-Onset Leukoencephalopathy and Can Result in Congenital Absence of Microglia. Am J Hum Genet. 2019 Mar 29; PubMed.
Oosterhof N, Kuil LE, van der Linde HC, Burm SM, Berdowski W, van Ijcken WF, van Swieten JC, Hol EM, Verheijen MH, van Ham TJ. Colony-Stimulating Factor 1 Receptor (CSF1R) Regulates Microglia Density and Distribution, but Not Microglia Differentiation In Vivo. Cell Rep. 2018 Jul 31;24(5):1203-1217.e6. PubMed.
Wlodarczyk A, Holtman IR, Krueger M, Yogev N, Bruttger J, Khorooshi R, Benmamar-Badel A, de Boer-Bergsma JJ, Martin NA, Karram K, Kramer I, Boddeke EW, Waisman A, Eggen BJ, Owens T. A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 2017 Nov 15;36(22):3292-3308. Epub 2017 Sep 28 PubMed.
Hagemeyer N, Hanft KM, Akriditou MA, Unger N, Park ES, Stanley ER, Staszewski O, Dimou L, Prinz M. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 2017 Sep;134(3):441-458. Epub 2017 Jul 6 PubMed.
Bennett FC, Bennett ML, Yaqoob F, Mulinyawe SB, Grant GA, Hayden Gephart M, Plowey ED, Barres BA. A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron. 2018 Jun 27;98(6):1170-1183.e8. Epub 2018 May 31 PubMed.
van Rappard DF, Boelens JJ, van Egmond ME, Kuball J, van Hasselt PM, Oostrom KJ, Pouwels PJ, van der Knaap MS, Hollak CE, Wolf NI. Efficacy of hematopoietic cell transplantation in metachromatic leukodystrophy: the Dutch experience. Blood. 2016 Jun 16;127(24):3098-101. Epub 2016 Apr 26 PubMed.
University of California, Irvine
This paper, combined with the Monje lab’s recent Cell paper (Gibson et al., 2018), elegantly highlights the complexity of cellular interactions in toxicity/disease states, and place microglia as the major effector cell type that has broad effects on astrocytes, neurons, and oligodendrocytes following chemotherapy.
The previous paper demonstrated that MTX treatment led to changes in microglial activation states that in turn regulated astrocyte signaling and function, ultimately causing deficits in myelination and leading to cognitive impairments. This follow-up study identifies how MTX-mediated microglial changes downregulate neuronal BDNF levels, which in turn reduce activity-dependent myelination via inhibition of OPC proliferation. Notably, depletion of microglia via the CSF1R inhibitor PLX5622 reversed all the phenotypes associated with MTX administration (cognitive impairments, changes in astrocyte phenotypes, myelination, and reductions in BDNF).
Overall, these results shed light on the upstream causes of "chemo-brain" and suggest that targeting of microglia would be broadly beneficial. The challenge will be on how to target microglia in the human brain, as microglial depletion in humans is probably premature.
Of relevance to Alzheimer's disease and other brain disorders, these studies show that cells do not work alone in the brain, but have highly complex relationships with one another, and that understanding and dissecting these relationships is challenging but critical. How glial cells can regulate neuronal form and function may be integral to the pathogenesis of diseases such as AD.
References:
Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L, Woo PJ, Barres BA, Liddelow S, Vogel H, Monje M. Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell. 2019 Jan 10;176(1-2):43-55.e13. Epub 2018 Dec 6 PubMed.
Make a Comment
To make a comment you must login or register.