X-Chromosome-Wide Association Data Spot Alzheimer’s Loci
Quick Links
About two-thirds of people with Alzheimer’s disease are women, yet due to the challenges in studying X chromosomes, a genetic foundation for this has been difficult to uncover. Now, two large X-chromosome-wide association studies (XWAS) and a smaller one based on pathologically confirmed AD identify 12 loci that might shed some light on the matter.
- One study included data from more than 1 million people.
- It pegged a variant in SLC9A7 that boosts AD risk by 5 percent.
- The variant may ramp up Aβ production by disrupting pH in the Golgi.
In the September 9 JAMA Neurology, scientists led by Michael Belloy, Washington University in St. Louis, report four common and two rare variants on the X chromosome that associate with AD. This follows an XWAS uploaded to medRxiv May 3, in which an international group led by Céline Bellenguez, University of Lille, France, identified four different loci, and a paper published September 4 in Translational Psychiatry describing two more that were uncovered by Valentina Escott-Price, Cardiff University, U.K., and her colleagues.
Of these 12 loci, only one, near the SLC9A7 gene, reached genome-wide significance. The other 11 were significant at the chromosome-wide level and warrant further study, scientists say. In association studies, scientist consider variants that pass genome-wide significance as “hits,” and those that pass the less-stringent chromosome-wide test as “suggestive.”
“The new XWASes and other X chromosome studies are pivotal because they could pave the way for new therapeutic targets that benefit men, women, or both sexes,” wrote Dena Dubal of the University of California, San Francisco, who was not involved in any of the studies.
The X chromosome contains about 1,200 genes. “This is 5 percent of the genome that has been dark to us,” Belloy told Alzforum. This chromosome is typically omitted from GWAS because it complicates statistical analyses. In women, most genes on one of these two chromosomes are inactivated during early embryonic development to avoid double-dosing. Some genes escape this inactivation, however, and this is unpredictable and can change during life (see Peeters et al., 2023, for a review).
Belloy and his colleagues parsed the genomes of more than 1,100,000 people, including women and men with AD, their first-degree relatives, as well as healthy controls, to look for variants that associate with AD. The dataset, which included cohorts from the Alzheimer’s Disease Genetics Consortium, Alzheimer’s Disease Sequencing Project, U.K. Biobank (UKB), Finnish health registry (FinnGen), and U.S. Million Veterans Program (MVP), yielded six independent genetic variants—four could be tied to the regulation of nearby genes.
Of the six, one, a single-nucleotide variation in an intron of SLC9A7 stood out. The variant not only exceeded the statistical significance threshold for genome-wide association, but, mechanistically, the SLC9A7 gene might promote Aβ production.

X Factors. Manhattan plot of XWAS meta-analysis shows two rare and four common variants linked to AD. SLC9A7 passed a conservative genome-wide significance threshold (upper horizontal line). The others passed the threshold for X-chromosome-wide significance, a less stringent bar (lower line). [Courtesy of Belloy et al., 2024.]
Previously, scientists led by coauthor Joachim Herz at the University of Texas Southwestern Medical Center in Dallas had reported that greater expression of the sodium-hydrogen exchanger NHE6, encoded by the SLC9A7 ortholog SLC9A6, triggers ApoE aggregation, which in turn leads to Aβ accumulation (see Jul 2021 news; Pohlkamp et al., 2021). NHEs function in cellular homeostasis. Blocking NHE6 restored acidification of the early endosomal compartment and suppressed amyloid accumulation, Herz found.
Both SLC9A6 and SLC9A7 are highly conserved across species. “Although their functions aren't exactly the same, they are closely related,” Belloy said. These exchangers regulate the pH and ion levels in their respective cellular compartments—early endosomes for NHE6, the Golgi for NHE7. The researchers suspect that an increase in NHE7 would disrupt the pH in the Golgi compartment and that this, too, could lead to amyloidosis.
How big an effect might the SLC9A7 variant have on AD risk? Because X chromosome inactivation complicates the analyses, Belloy and colleagues came up with a statistical method to account for it. This model suggested that an active SLC9A7 risk variant could nudge up expression of the gene in brain tissue, but only by 16 to 44 percent. Though this seems small, the clinical relevance may be greater, Belloy said. “We think that in early life, you can tolerate a small difference in expression of this gene, but as you age, that becomes more of an issue and can contribute to a build-up of amyloid and tau pathology,” he said. The variant associated with AD in both women and men.
Also X inactivated in women is MTM1, a locus that just missed genome-wide statistical significance. In contrast, the four other loci that passed X chromosome-wide significance—NLGN4X, MID1, ZNF280C, and ADGRG4—appear to escape X inactivation. Therefore, they are likely to affect women more than men.
Bellenguez and colleagues analyzed data from 115,841 AD or AD-proxy cases and 613,671 controls that they obtained from the International Genomics of Alzheimer’s Project, European Alzheimer & Dementia Biobank, UKB, and FinnGen. First authors Julie Le Borgne and Lissette Gomez identified four loci that reached X chromosome-wide, though again not genome-wide, significance. Two fell in introns of genes that are X-inactivated, namely FRMPD4 and DMD. The former regulates dendritic spines and neurotransmission and variants in it cause X-linked intellectual disability. Variants in the adjacent gene MSL3, which is not subject to X-inactivation, cause neurodevelopmental delay (Piard et al., 2018; Basilicata et al., 2018).
Variants in DMD, which encodes dystrophin, cause Duchenne muscular dystrophy, which mostly affects boys, and about one-third of them also have cognitive impairment. In male mice, DMD mutations may trigger amyloidosis in the prefrontal cortex and hippocampus (Hayward et al., 2022).
Functional effects for the other two loci are difficult to predict, the authors say, since they both are more than 300bp away from the nearest gene, namely NLGN4X and GRIA3. Dubal previously tied GRIA3 expression to cognitive resilience in women, but the locus Borgne and colleagues identified only associated with AD in men (Davis et al., 2021).
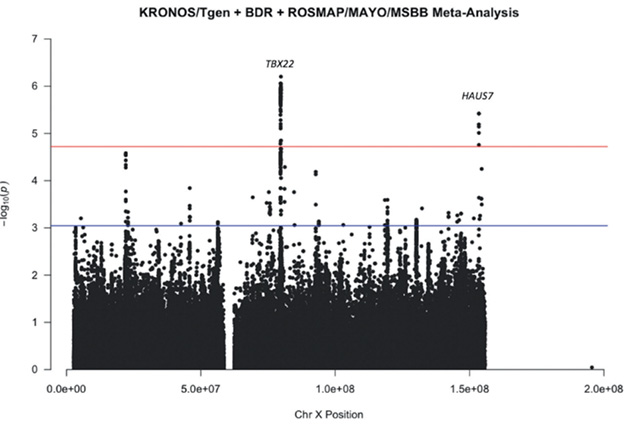
AD Loci? Variants near the TBX22 and Haus7 genes reached the chromosome-wide significance threshold in an XWAS meta-analyses of pathologically confirmed AD. [Courtesy of Escott-Price et al., 2024]
Though their study was smaller, Escott-Price and her colleagues included data from 1,970 people who had had pathologically confirmed AD from 1,113 controls. First author Emily Simmonds analyzed datasets from three tissue bank cohorts: Brains for Dementia Research UK; a KRONOS/Tgen dataset derived from 21 National Alzheimer’s Coordinating Center brain banks and the Miami Brain Bank; and a combination of the Religious Orders Study/Memory and Aging Project, Mount Sinai Brain Bank, and Mayo Clinic Brain Bank. The analysis yielded 264,793 SNPs, of which two, at the TBX22 and Haus7 loci, reached chromosome-wide significance in meta-analysis of all three cohorts, while one, NXF5, passed muster in women only. The authors were intrigued by TBX22 and three other candidate genes, DDX53, IL1RAPL1, and SH3BGRL because all four turned up in separate analysis of at least two of the three cohorts.
Anomalies in three of these genes have previously been reported in various AD models, including elevated expression of DDX53, suppression of IL1RAPL1, and evidence of both in SH3BGRL. NXF5 deficiency has been linked to intellectual disabilities (Callaerts-Vegh et al., 2015).
“Collectively, these studies highlight our emerging and high value for the X chromosome as a contributor to neural-related functions and as a source of sex difference,” Dubal said.
Both Belloy and Bellenguez suspect that variants in genes that escape inactivation help explain the sex differences in AD seen clinically. Women, for example, have less cognitive resilience to AD pathology, as determined by amyloid PET, than men. And yet, they have greater brain resilience to the effects of tangles than men (see Arenaza-Urquijo et al., 2024, for a review).
Teasing out such effects with XWAS alone may be difficult. Other X chromosome-related factors may also play a role in AD, including epigenetic alterations of the X, genomic imprinting, and X-linked proteomic signatures, noted Rachel Buckley and Mabel Seto, Brigham & Women’s Hospital, Boston, in a JAMA Neurology editorial. “None of these components are effectively captured by XWAS,” they wrote.
Belloy agreed that these phenomena are grist for future analyses. “Our study will allow us to really start exploring how the X chromosome may be implicated in sex differences in Alzheimer’s disease,” he said. “This was a first step.”—Kristel Tjandra
Kristel Tjandra is a freelance writer in Springfield, Virginia.
References
News Citations
Paper Citations
- Peeters SB, Posynick BJ, Brown CJ. Out of the Silence: Insights into How Genes Escape X-Chromosome Inactivation. Epigenomes. 2023 Nov 23;7(4) PubMed.
- Pohlkamp T, Xian X, Wong CH, Durakoglugil MS, Werthmann GC, Saido TC, Evers BM, White CL 3rd, Connor J, Hammer RE, Herz J. NHE6 depletion corrects ApoE4-mediated synaptic impairments and reduces amyloid plaque load. Elife. 2021 Oct 7;10 PubMed.
- Piard J, Hu JH, Campeau PM, Rzonca S, Van Esch H, Vincent E, Han M, Rossignol E, Castaneda J, Chelly J, Skinner C, Kalscheuer VM, Wang R, Lemyre E, Kosinska J, Stawinski P, Bal J, Hoffman DA, Schwartz CE, Van Maldergem L, Wang T, Worley PF. FRMPD4 mutations cause X-linked intellectual disability and disrupt dendritic spine morphogenesis. Hum Mol Genet. 2018 Feb 15;27(4):589-600. PubMed.
- Basilicata MF, Bruel AL, Semplicio G, Valsecchi CI, Aktaş T, Duffourd Y, Rumpf T, Morton J, Bache I, Szymanski WG, Gilissen C, Vanakker O, Õunap K, Mittler G, van der Burgt I, El Chehadeh S, Cho MT, Pfundt R, Tan TY, Kirchhoff M, Menten B, Vergult S, Lindstrom K, Reis A, Johnson DS, Fryer A, McKay V, DDD Study, Fisher RB, Thauvin-Robinet C, Francis D, Roscioli T, Pajusalu S, Radtke K, Ganesh J, Brunner HG, Wilson M, Faivre L, Kalscheuer VM, Thevenon J, Akhtar A. De novo mutations in MSL3 cause an X-linked syndrome marked by impaired histone H4 lysine 16 acetylation. Nat Genet. 2018 Oct;50(10):1442-1451. Epub 2018 Sep 17 PubMed.
- Hayward GC, Caceres D, Copeland EN, Baranowski BJ, Mohammad A, Whitley KC, Fajardo VA, MacPherson RE. Characterization of Alzheimer's disease-like neuropathology in Duchenne's muscular dystrophy using the DBA/2J mdx mouse model. FEBS Open Bio. 2022 Jan;12(1):154-162. Epub 2021 Nov 11 PubMed.
- Davis EJ, Solsberg CW, White CC, Miñones-Moyano E, Sirota M, Chibnik L, Bennett DA, De Jager PL, Yokoyama JS, Dubal DB. Sex-Specific Association of the X Chromosome With Cognitive Change and Tau Pathology in Aging and Alzheimer Disease. JAMA Neurol. 2021 Oct 1;78(10):1249-1254. PubMed.
- Callaerts-Vegh Z, Ahmed T, Vermaercke B, Marynen P, Balschun D, Froyen G, D'Hooge R. Nxf7 deficiency impairs social exploration and spatio-cognitive abilities as well as hippocampal synaptic plasticity in mice. Front Behav Neurosci. 2015;9:179. Epub 2015 Jul 10 PubMed.
- Arenaza-Urquijo EM, Boyle R, Casaletto K, Anstey KJ, Vila-Castelar C, Colverson A, Palpatzis E, Eissman JM, Kheng Siang Ng T, Raghavan S, Akinci M, Vonk JM, Machado LS, Zanwar PP, Shrestha HL, Wagner M, Tamburin S, Sohrabi HR, Loi S, Bartrés-Faz D, Dubal DB, Vemuri P, Okonkwo O, Hohman TJ, Ewers M, Buckley RF, Reserve, Resilience and Protective Factors Professional Interest Area, Sex and Gender Professional Interest area and the ADDRESS! Special Interest Group. Sex and gender differences in cognitive resilience to aging and Alzheimer's disease. Alzheimers Dement. 2024 Aug;20(8):5695-5719. Epub 2024 Jul 5 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Belloy ME, Le Guen Y, Stewart I, Williams K, Herz J, Sherva R, Zhang R, Merritt V, Panizzon MS, Hauger RL, Gaziano JM, Logue M, Napolioni V, Greicius MD. Role of the X Chromosome in Alzheimer Disease Genetics. JAMA Neurol. 2024 Oct 1;81(10):1032-1042. PubMed.
- LeBorgne J, Gomez L, Heikkinen S, Amin N, Ahmad S, Choi SH, Bis J, Grenier-Boley B, Rodriguez OG, Kleineidam L, Young J, Tripathi KP, Wang L, Varma A, vanderLee S, Damotte V, deRojas I, Palmal S, EADB, GR@ACE, DEGESCO, EADI, GERAD, DemGene, FinnGen, ADGC, CHARGE, Giedraitis V, Ghidoni R, Fernandez V, Kehoe PG, Frikke-Schmidt R, Tsolaki M, Sanchez-Juan P, Sleegers K, Ingelsson M, Haines J, Farrer L, Mayeux R, Wang L-S, Sims R, DeStefano A, Schellenberg GD, Seshadri S, Amouyel P, Wil. X-chromosome-wide association study for Alzheimer's disease. 2024 May 03 10.1101/2024.05.02.24306739 (version 1) medRxiv.
- Simmonds E, Leonenko G, Yaman U, Bellou E, Myers A, Morgan K, Brookes K, Hardy J, Salih D, Escott-Price V. Chromosome X-wide association study in case control studies of pathologically confirmed Alzheimer's disease in a European population. Transl Psychiatry. 2024 Sep 4;14(1):358. PubMed.
- Buckley RF, Seto M. How Is the X Chromosome Involved in Alzheimer Disease?. JAMA Neurol. 2024 Oct 1;81(10):1028-1029. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.