Viral Vectors Trigger Robust Tauopathy in Brain Slices
Quick Links
Research on neurodegenerative disease is advancing slowly in part because most models aren’t very good. In the February 15 Journal of Experimental Medicine, researchers led by Todd Golde at the University of Florida, Gainesville, debuted a new system for studying proteinopathies in a dish. They transduced mouse brain slices with genes for mutant human tau, and produced abundant neurofibrillary tangle pathology within one month. Overexpression of human α-synuclein sparked Lewy body formation in the same time frame. In addition, the researchers used different promoters to target expression to specific types of brain cell. The system offers a fast, versatile option for drug screening and mechanistic studies of neurodegenerative disease, the authors suggested.
- Viral transduction of mutant human tau produces robust tauopathy in mouse brain slices.
- Transduction of human α-synuclein gives rise to Lewy bodies.
- Model system may accelerate drug testing and mechanistic studies.
Peter Reinhart at the University of Massachusetts, Amherst, agreed. “This opens the door for new types of studies,” he wrote to Alzforum.
Research on mouse models is not only slow; it’s also expensive. Cell cultures, while offering faster testing, contain neither all the cell types nor the architecture present in the brain, and thus often fail to predict what will happen in vivo. Golde and colleagues wondered if slice cultures could represent a happy medium.
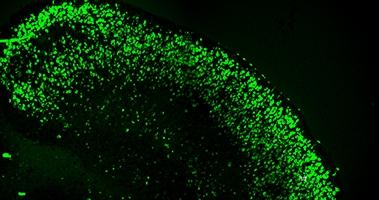
Induced Tangles. Mouse brain slices transduced with a human tau gene carrying A152T, P301L, and S320F mutations develop tau tangles as judged by Thioflavin S staining (green) by 28 days in vitro. [Courtesy of Cara Croft and Todd Golde.]
To culture slices, first author Cara Croft dissected the cortices, hippocampi, and connecting regions from eight-day-old wild-type mice, then cut them into 350 μm thick coronal slices. The scientists transduced the slices with recombinant adeno-associated viruses (rAAV). They compared different types of protein coat packaging, or capsids, to identify those that produced the highest gene expression. Distinct promoters restricted transgene expression to neurons, oligodendrocytes, astrocytes, or microglia. Transgene expression lasted from three to nine months.
Could this work to model tauopathy? Using a general promoter that targets all cells, the authors transduced the slices with several different human tau genes: wild-type, the pro-aggregant S320F mutation, a S320F/P301L, and a S320F/P301L/A152T combination. All of these bumped up the abundance of phosphorylated tau after 28 days in culture over vector control, but only S320F/P301L and S320F/P301L/A152T also caused tau inclusions in cell bodies. The deposits stained with Thioflavin S, and electron microscopy confirmed the presence of tau filaments, indicating these were bona fide neurofibrillary tangles. The tangles accumulated throughout the brain slice, with thousands apparent after one month in culture. At this time point, rates of cell death were the same as in control slices; however, by 60 days, about three times as many dead cells had accumulated in the tauopathy slices as in controls.
This slice model could be used to screen drugs and predict their likely in-vivo efficacy, the authors suggested. As a proof of concept, they tested four different GSK3β inhibitors on the tauopathy slice cultures from day 14 to 28. The GSK3β enzyme phosphorylates tau, but only one of the four inhibitor drugs suppressed paired helical filaments and tangles in this milieu. This underlines the idea that only some therapies will work in the brain, the authors noted.
In ongoing work, the authors are examining how tangles change over time and how they might trigger cell death, as well as how different mutations affect seeding. They are collaborating with academic and industry researchers to test potential tau therapeutics in this system. “Our goal is to stress-test this system to see if it is predictive of effects in tau mice,” Croft and Golde wrote to Alzforum.
Could these slice cultures model other protein-aggregation diseases as well? Perhaps. Slices transduced with human wild-type or A53T α-synuclein developed Lewy bodies by 28 days in vitro, and immunostaining and electron microscopy confirmed the presence of α-synuclein fibrils. The authors are collaborating to establish slice models of additional neurodegenerative diseases.
“Using rAAV promoter-capsid combinations, we can flexibly and cost-effectively manipulate expression of genes in all the major central-nervous-system cell types in organotypic brain slice cultures in a manner which was previously not possible. This will no doubt accelerate many research studies in neurodegenerative proteinopathies and likely be leveraged by neuroscientists in many research areas,” they wrote.—Madolyn Bowman Rogers
References
No Available References
Further Reading
Primary Papers
- Croft CL, Cruz PE, Ryu DH, Ceballos-Diaz C, Strang KH, Woody BM, Lin WL, Deture M, Rodríguez-Lebrón E, Dickson DW, Chakrabarty P, Levites Y, Giasson BI, Golde TE. rAAV-based brain slice culture models of Alzheimer's and Parkinson's disease inclusion pathologies. J Exp Med. 2019 Mar 4;216(3):539-555. Epub 2019 Feb 15 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Massachusetts General Hospital
Massachusetts General Hospital
Massachusetts General Hospital
This study describes a toolkit for studying neurofibrillary inclusion and Lewy body formation in an in vivo-like murine environment. Organotypic brain slice cultures have been used to study brain cells ex vivo for more than a decade. Multiple tauopathy and AD murine brain slice culture (BSC) models have been previously reported, most of which used the established tauopathy or AD transgenic mice (Croft and Noble, 2018; Croft et al., 2017; Harwell and Coleman, 2016; Humpel, 2015; Messing et al., 2013; Mewes et al., 2012; Benediktsson et al., 2005).
Here, Drs. Croft and Golde describe mouse BSC models of neurofibrillary inclusion and Lewy body formation using rAAVs that express tau or α-synuclein variants in different brain cell types. They showed that overexpression of combinations of mutations leading to tau aggregation (S320F/P301L and S320F/P301L/A152T) induced robust Thio-S positive and Sarkosyl-insoluble tau aggregates in 28 days of culture. Importantly, these mutations are not directly associated with Alzheimer's disease, and accelerated tau aggregation owing to FTLD mutations would confound the elucidation of upstream pathogenic events linking Aβ deposition to tau aggregation in AD.
Previously, human iPSC-derived neural cell cultures from AD patients have been shown to undergo accumulation of hyperphosphorylated tau proteins (Van Der Kant et al., 2019; Ovchinnikov et al., 2018; Ochalek et al., 2017; Raja et al., 2016; Moore et al., 2015; Muratore et al., 2014; Shi et al., 2012; Israel et al., 2012). However, as Croft et al. point out, it is difficult to obtain actual tau inclusion pathology in human iPSC-derived neural cell cultures.
Along these lines, Croft et al. referenced our study (Choi et al., 2014). We employed human neuronal progenitor cells (RenCell VM™ originally derived from human fetal brain), infected with Lentiviral constructs expressing APP/PS1 with FAD mutations, and we grew these cells in a three-dimensional system. This led to robust Aβ and tau pathology, including not only the accumulation of hyperphosphorylated tau in dendrites and cell bodies, but also detergent-insoluble tau species with tangle-like filamentous structure, as shown by EM. Importantly, this pathology was achieved without overexpressing either wild-type or mutant tau.
In an effort to improve our three-dimensional models, in collaboration with Dr. Cho’s lab at UNCC, we recently reported a three-dimensional human neuron-astrocyte-microglia triculture model that can recapitulate robust neurons death and inflammation in human AD brain-like environment (Park et al., 2018). To date, filamentous tau tangle-like structures have not been achieved in other human neural cell culture models.
We strongly believe that our three-dimensional human cell culture models for AD, as well as Dr. Croft’s new rAAV BSC tau murine models employing overexpression of FTLD-associated tau mutations (and an α-synuclein mutation), should facilitate the search for novel therapeutic target for AD and related diseases.
References:
Croft CL, Noble W. Preparation of organotypic brain slice cultures for the study of Alzheimer's disease. F1000Res. 2018;7:592. Epub 2018 May 15 PubMed.
Croft CL, Wade MA, Kurbatskaya K, Mastrandreas P, Hughes MM, Phillips EC, Pooler AM, Perkinton MS, Hanger DP, Noble W. Membrane association and release of wild-type and pathological tau from organotypic brain slice cultures. Cell Death Dis. 2017 Mar 16;8(3):e2671. PubMed.
Harwell CS, Coleman MP. Synaptophysin depletion and intraneuronal Aβ in organotypic hippocampal slice cultures from huAPP transgenic mice. Mol Neurodegener. 2016 Jun 10;11(1):44. PubMed.
Humpel C. Organotypic vibrosections from whole brain adult Alzheimer mice (overexpressing amyloid-precursor-protein with the Swedish-Dutch-Iowa mutations) as a model to study clearance of beta-amyloid plaques. Front Aging Neurosci. 2015;7:47. Epub 2015 Apr 9 PubMed.
Messing L, Decker JM, Joseph M, Mandelkow E, Mandelkow EM. Cascade of tau toxicity in inducible hippocampal brain slices and prevention by aggregation inhibitors. Neurobiol Aging. 2013 May;34(5):1343-54. PubMed.
Mewes A, Franke H, Singer D. Organotypic brain slice cultures of adult transgenic P301S mice--a model for tauopathy studies. PLoS One. 2012;7(9):e45017. PubMed.
Benediktsson AM, Schachtele SJ, Green SH, Dailey ME. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J Neurosci Methods. 2005 Jan 30;141(1):41-53. PubMed.
van der Kant R, Langness VF, Herrera CM, Williams DA, Fong LK, Leestemaker Y, Steenvoorden E, Rynearson KD, Brouwers JF, Helms JB, Ovaa H, Giera M, Wagner SL, Bang AG, Goldstein LS. Cholesterol Metabolism Is a Druggable Axis that Independently Regulates Tau and Amyloid-β in iPSC-Derived Alzheimer's Disease Neurons. Cell Stem Cell. 2019 Mar 7;24(3):363-375.e9. Epub 2019 Jan 24 PubMed.
Ovchinnikov DA, Korn O, Virshup I, Wells CA, Wolvetang EJ. The Impact of APP on Alzheimer-like Pathogenesis and Gene Expression in Down Syndrome iPSC-Derived Neurons. Stem Cell Reports. 2018 Jul 10;11(1):32-42. Epub 2018 May 31 PubMed.
Ochalek A, Mihalik B, Avci HX, Chandrasekaran A, Téglási A, Bock I, Giudice ML, Táncos Z, Molnár K, László L, Nielsen JE, Holst B, Freude K, Hyttel P, Kobolák J, Dinnyés A. Neurons derived from sporadic Alzheimer's disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation. Alzheimers Res Ther. 2017 Dec 1;9(1):90. PubMed.
Raja WK, Mungenast AE, Lin YT, Ko T, Abdurrob F, Seo J, Tsai LH. Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer's Disease Phenotypes. PLoS One. 2016;11(9):e0161969. Epub 2016 Sep 13 PubMed.
Moore S, Evans LD, Andersson T, Portelius E, Smith J, Dias TB, Saurat N, McGlade A, Kirwan P, Blennow K, Hardy J, Zetterberg H, Livesey FJ. APP metabolism regulates tau proteostasis in human cerebral cortex neurons. Cell Rep. 2015 May 5;11(5):689-96. Epub 2015 Apr 23 PubMed.
Muratore CR, Rice HC, Srikanth P, Callahan DG, Shin T, Benjamin LN, Walsh DM, Selkoe DJ, Young-Pearse TL. The familial Alzheimer's disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum Mol Genet. 2014 Jul 1;23(13):3523-36. Epub 2014 Feb 12 PubMed.
Shi Y, Kirwan P, Smith J, Maclean G, Orkin SH, Livesey FJ. A human stem cell model of early Alzheimer's disease pathology in Down syndrome. Sci Transl Med. 2012 Mar 7;4(124):124ra29. PubMed.
Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, Carson CT, Laurent LC, Marsala M, Gage FH, Remes AM, Koo EH, Goldstein LS. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature. 2012 Jan 25;482(7384):216-20. PubMed.
Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D'Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, Klee JB, Zhang C, Wainger BJ, Peitz M, Kovacs DM, Woolf CJ, Wagner SL, Tanzi RE, Kim DY. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature. 2014 Nov 13;515(7526):274-8. Epub 2014 Oct 12 PubMed.
Park J, Wetzel I, Marriott I, Dréau D, D'Avanzo C, Kim DY, Tanzi RE, Cho H. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer's disease. Nat Neurosci. 2018 Jul;21(7):941-951. Epub 2018 Jun 27 PubMed.
Make a Comment
To make a comment you must login or register.