Toward an AD CRISPR Therapy: Tweaking APP Terminus Cuts Plaque
Quick Links
When in the clutches of β-secretase, the amyloid precursor protein (APP) lives up to its name, churning out the starting material for Aβ peptides and all that comes from them. What if this whole fiasco could be avoided by keeping the two apart? A manuscript posted on bioRxiv June 9 gives credence to this strategy.
- Delivered by a virus, CRISPR pulled an endosomal targeting motif off APP in mouse brain.
- This shifted APP processing away from β-secretase cleavage.
- Plaque growth, lysosomal dysfunction, gliosis, and functional decline slowed for months.
Researchers led by Subhojit Roy at the University of California, San Diego, used CRISPR gene editing to remove a six-residue motif that targets APP to the endosome, where β-secretase, aka BACE1, lies in wait. In APP knock-in mice infected with a virus carrying CRISPR machinery, APP processing shifted from this amyloidogenic path toward α-secretase cleavage instead. Predictably, this reduced the Aβ plaque burden, inflammation, synaptic deficits, and memory loss.
The authors believe this gene therapy approach holds promise for people with familial, or even sporadic, Alzheimer’s disease. Participants of the dominantly inherited Alzheimer’s Network, aka DIAN, have been clamoring for gene therapy approaches to fix their disease; this approach represents one such attempt.
“A universal one-and-done gene therapy for AD, which the authors have set as their long-term goal, is alluring, especially in a field that has struggled for decades to find a suitable therapy that will stop the disease process in its tracks,” wrote Justyna Dobrowolska Zakaria of Northwestern University in Chicago. Still, she said a lot more work needs to be done before bringing the gene-editing therapy to the clinic, starting with perfecting delivery into the human brain.
While Aβ-targeted therapies can mop up existing aggregates, other strategies have sought to curb Aβ production. One such approach, BACE1 inhibition, fell flat in trials due to cognitive side effects, though some scientists propose trying again with lower drug doses (Jul 2023 conference news). Another approach is to quash translation of APP with antisense nucleotides, though that comes at the expense of its physiological function (Nov 2023 conference news). Roy’s approach avoids these pitfalls by keeping BACE and APP apart. Previously, the scientists had reported that clipping off the final exon of APP in human neurons yielded a truncated protein that lingered on the cell surface, where it was mostly processed by α-secretase (Sun et al., 2019). The exon contains the YENPTY motif that guides the precursor to endosomes.
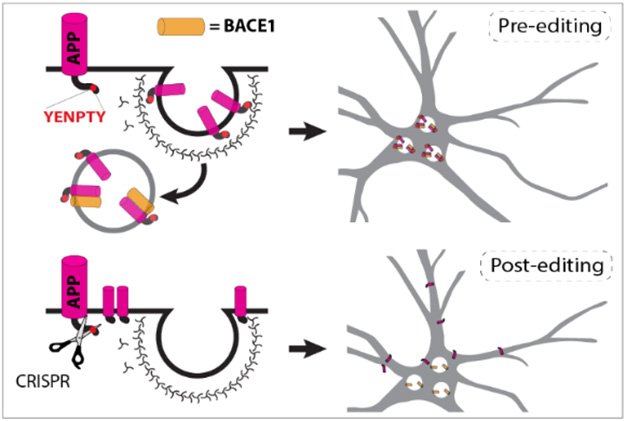
Keep Away. The YENPTY motif at APP’s C-terminus routes it to the endosome, where it is exposed to BACE1 cleavage (top). After CRISPR editing, APP stays on the cell surface, safely apart from BACE1 (bottom). [Courtesy of Aulston et al., bioRxiv, 2024.]
In their new study, first author Brent Aulston and colleagues tested this in APPNL-G-F knock-in mice, which start to develop plaques by 2 months of age. They crossed these to mice expressing Cas9 in the brain, the endonuclease that carries out CRISPR’s DNA snipping. To target APP’s final exon, the scientists packaged a guide RNA into an AAV vector optimized to cross the mouse blood-brain barrier.
After Aulston injected it into the blood, the virus broadly infected the brain. Editing worked with 90 percent efficiency in neurons, where most of the APP lacked the final 18 amino acids. Levels of sAPPα, a soluble product of α-secretase, were 25 percent higher, while sAPPβ was 25 percent lower than in mice treated with a nonspecific guide RNA. This suggested that the editing indeed sequestered APP from BACE1.
How about Aβ peptides? Ten months after infection, levels of insoluble Aβ42 and Aβ40 were half those in controls. This translated into less plaque. The difference grew over time as APPNL-G-F mice continued to lay down amyloid while APP-edited mice did so more slowly (image below).

Avoid Amyloidosis. Ten months after APP-KI/Cas9 mice were infected with virus carrying an APP-editing guide RNA, they had less amyloid plaque (right) than did mice infected with a control guide RNA (left). [Courtesy of Aulston et al., 2024.]
Cutting off APP’s last exon countered disease phenotypes in the APP-KI mice. Enlarged lysosomes, microgliosis and astrocytosis, and synaptic function were less pathological, as was memory loss gauged by how long mice explored a novel object.
Roy thinks the benefits of this editing might stem not only from Aβ reduction. There’s also a drop in β-CTF, a β-secretase product reported to exacerbate lysosomal dysfunction (Aug 2019 news). A study from Ralph Nixon’s lab at New York University claims the YENPTY motif in β-CTF derails lysosomal acidification (Jul 2023 news).
In other experiments, Roy’s group edited APP in the knock-in mice from birth. This almost completely prevented formation of Aβ plaques, particularly in mice expressing only one copy of the NL-G-FAPP variant, i.e., mice that better reflect the heterozygous nature of most familial AD mutations. Single-nucleus transcriptomic analysis in these mice indicated that their microglia remained in a homeostatic state at 10 months of age, at which point these cells had transitioned into a disease-associated (DAM) state in controls.
This editing strategy worked in Cas9 knock-ins, but neither mice nor people express this bacterial gene. Hence the scientists packaged a slimmed-down version of the endonuclease, called SA-Cas9, into the AAV vector along with the APP-targeted guide RNA. Infecting APP-KI mice with that similarly reduced amyloidosis, microgliosis, and lysosomal dysfunction. Ongoing work in Roy’s lab seeks to optimize the guide RNAs, Cas9 endonuclease, and AAV vectors for use in people.
Martin Ingelsson of the University of Toronto pointed out that the gene editing was initiated slightly before plaques start to accumulate, and the mice were sacrificed slightly before they would have had saturated plaque levels in their brains. “To get closer to a ‘real-world clinical scenario,’ it would be relevant to also treat older mice for a more limited period,” Ingelsson wrote (comment below).
While not in the manuscript, Roy told Alzforum that these experiments—in 6-month-old mice—have recently been done. CRISPR editing did not eliminate existing plaques, but it did prevent more from forming, he said. He said the Arctic mutation in these knock-ins yields Aβ plaques that are particularly sticky and stubborn, so their persistence even after APP editing did not surprise him.
Who could be candidates for this gene therapy? Roy thinks it could work for people who have one of the hundreds of known ADAD mutations in APP, presenilin 1, or PS2. To test this idea, he is comparing how well the editing works across human isogenic iPSC-derived neurons from different ADAD mutations.
Eric McDade of Washington University in St. Louis thinks this cell work is critical. “If the outcomes are similar, this could overcome a significant problem of having to target each of the ADAD mutations individually,” he wrote. McDade, a lead investigator in DIAN, was excited to see research move a step closer to use in humans. “This is an approach that many individuals with ADAD mutations have expressed a high level of enthusiasm for,” he wrote (comment below).
Roy said that people with ADAD mutations make ideal candidates for initial human studies. In the future, he envisions using APP editing in people with sporadic AD as well, perhaps in combination with Aβ-targeted antibodies. “After removal of existing amyloid, this one-time gene editing therapy could turn off the tap,” he said.
Kristine Freude of the University of Copenhagen thinks the gene editing elegantly thwarted Aβ-related pathology in mice. “However, translating these findings to humans poses challenges, including delivery methods and the presence of tau pathology,” she wrote. “Investigating the impact of CRISPR/Cas9-mediated APP modifications on tau pathology in AD would be highly relevant.”
Roy’s AAV- and CRISPR-based approach comes at a time when both technologies are making strides in clinical studies. In a first, the FDA approved a CRISPR therapy for sickle-cell anemia last December after approving the AAV-based spinal muscular atrophy therapy Zolgemsa back in 2019 (FDA press release; Nov 2019 news). Other AAV-based biologics are in preclinical and early clinical studies for Gaucher’s, frontotemporal dementia, and other neurodegenerative diseases (Jun 2024 news). Roy thinks clinical studies of his approach are a realistic goal, and has founded the startup CRISPRAlz.—Jessica Shugart
References
News Citations
- Give BACE Inhibitors a Second Chance?
- Moving Forward: RNA-Targeted Attempts at Taking Down Tau, APP
- Familial AD Mutations, β-CTF, Spell Trouble for Endosomes
- Too Basic: APP β-CTF's YENTPY Motif Binds Proton Pump, Thwarts Lysosomes
- Time to Try Again: Gene-Based Therapy for Neurodegeneration
- All About Exposure: How to Get Enough Progranulin into the Brain?
Research Models Citations
Paper Citations
- Sun J, Carlson-Stevermer J, Das U, Shen M, Delenclos M, Snead AM, Koo SY, Wang L, Qiao D, Loi J, Petersen AJ, Stockton M, Bhattacharyya A, Jones MV, Zhao X, McLean PJ, Sproul AA, Saha K, Roy S. CRISPR/Cas9 editing of APP C-terminus attenuates β-cleavage and promotes α-cleavage. Nat Commun. 2019 Jan 3;10(1):53. PubMed.
Other Citations
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.