Test Tracks Preclinical Functional Decline
Quick Links
As the field inches toward treating Alzheimer’s disease at the preclinical stage, researchers need to find sensitive tests that can tell whether therapies work. Measures that pick up subtle cognitive decline in healthy elderly are coming along, but how to detect changes in people's everyday function is less clear. To this end, the Alzheimer’s Disease Cooperative Study (ADCS) has spent the last 10 years developing the Cognitive Function Instrument (CFI). In the February 23 JAMA Neurology, researchers led by Reisa Sperling at Brigham and Women’s Hospital, Boston, report that the CFI tracks longitudinal decline in people who start out cognitively normal, suggesting it may serve as a good functional outcome measure in prevention trials. “While cognitive outcomes are considered the gold standard, you want to show that a treatment impacts people’s everyday functioning,” said Rebecca Amariglio, first author on the paper. “That’s what matters most to people participating in these studies.”
The most recent draft guidance from the FDA suggests that a treatment can gain provisional approval for preclinical Alzheimer's based on a single cognitive measure, but will eventually have to demonstrate an improvement on function as well (see Mar 2013 news). Unfortunately, most of the tried-and-true functional measures, such as the ADCS Activities of Daily Living, do better at detecting drastic changes during the later stages of disease than subtle changes in people who may be on the cusp.
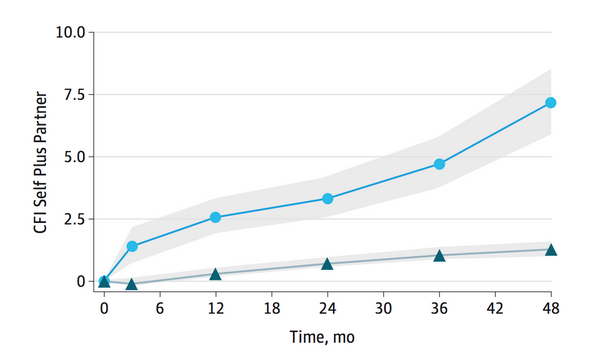
CFI Sets Groups Apart:
Scores on the CFI differentiated people who eventually progressed to MCI (circles) from those who stayed stable (triangles). Credit: Copyright © (2015) American Medical Association. All rights reserved.
The CFI survey is administered by mail, over the phone, or online (see paper). It is short, requires no interview, and is relatively inexpensive to use in large trials. Study participants and their partners each answer parallel questions separately, addressing early signs of problems with everyday functions, such as remembering appointments, driving, keeping track of finances, navigating around town, or following news or story plots. The survey also asks about perceived decline, such as whether the person's memory has worsened over the previous year, or whether they repeat questions. Responders answer yes, no, or maybe, to 14 questions to yield a score of 1, 0, or 0.5, respectively. Summed scores generated from the patient and his or her partner can be analyzed separately or together. By assessing subjective functional change, the test complements the modified ADCS Preclinical Alzheimer Cognitive Composite (mADCS-PACC), which detects objective cognitive changes in apparently healthy people (see Jun 2014 news). In the ongoing A4 trial the mADCS-PACC will be used as a primary, and the CFI as a secondary, outcome measure (see Dec 2014 conference story).
To test whether the CFI captures longitudinal change, Amariglio and colleagues enrolled 468 older individuals, average age 79, who were considered cognitively healthy based on the Clinical Dementia Rating Scale (CDR), modified mini-mental state examination (3MSE), and free and cued selective reminding test (FCSRT). During the four-year study, each participant came in for an annual cognitive assessment. A month before each visit, each completed the CFI survey. The researchers sent the parallel version to each person’s study partner. At the end of the study, the researchers correlated changing CFI scores with clinical progression, change on the mADCS-PACC, and ApoE4 status.
People who eventually became impaired, i.e., progressed to a CDR greater than 0, started out with higher CFI scores at baseline than people who remained stable over the four years of study. Since all began with a CDR of 0, it suggests that the survey detects functional problems before the CDR does. During the study, scores worsened on the self, partner, and combined tests for those who declined. The “self plus partner” score best predicted CDR change (see image above), implying that both inputs reinforce each other.
Overall, the scores correlated well with cognitive decline detected by the mADCS-PACC, with higher CFI tallies tracking with worsening cognition. Again, the combined score correlated best, followed by the partner- and self-assessment scores. Both mADCS-PACC and CFI predicted decline independently, but together performed slightly better, hinting that cognitive plus functional measures are most informative, wrote the authors. Since the researchers measured no biomarkers to confirm that Alzheimer's was behind these early changes, they used ApoE4 status as a proxy. While the differences were less stark than between progressors and non-progressors, the partner CFIs did differentiate ApoE carriers and non-carriers by three years into the study.
The CFI could provide an outcome measure in preventative drug trials for AD, allowing scientists to compare subjective cognitive function of treated and untreated groups, said Amariglio. In the future, researchers may also develop this test as a diagnostic tool to pick out people at risk for decline, but more research needs to determine the appropriate cutoffs, she said.
This is a really important contribution to the field,” said Jessica Langbaum of Banner Alzheimer's Institute in Phoenix. That the CFI requires no interview is a major plus, she added. Langbaum and other scientists of the Alzheimer’s Prevention Initiative (API) and Novartis are still deciding which patient-reported outcomes to use in the API ApoE4 trial. Langbaum said it would be interesting to compare the CFI with the more extensive ADCS measure of function, the Activities of Daily Living–Prevention Instrument, which does require a physician interview (see Galasko et al., 2006). The ADL-PI is longer and includes no questions about memory function, which may be important to detect early progression, wrote Amariglio and colleagues. It is also more difficult to score and interpret, said Amariglio. The correlation between CFI and ADL-PI questionnaires is in the range of 0.3 to 0.4, she said.
“There is without doubt a clear need for new measures of functional impairment,” John Harrison of Metis Cognition, Wiltshire, U.K., wrote to Alzforum (see full comment below). “With the interest in AD focused largely on secondary prevention, appropriate and sensitive measures of functional activities are essential. Of course, one of the big questions is whether these scales can capture the effect of pharmaceutical and other interventions."—Gwyneth Dickey Zakaib
References
News Citations
- FDA Invites Comment on Drug Testing Guidance for Early AD
- Test Battery Picks Up Cognitive Decline in Normal Populations
- From Shared CAP, Secondary Prevention Trials Are Off and Running
Paper Citations
- Walsh SP, Raman R, Jones KB, Aisen PS, . ADCS Prevention Instrument Project: the Mail-In Cognitive Function Screening Instrument (MCFSI). Alzheimer Dis Assoc Disord. 2006 Oct-Dec;20(4 Suppl 3):S170-8. PubMed.
- Galasko D, Bennett DA, Sano M, Marson D, Kaye J, Edland SD, . ADCS Prevention Instrument Project: assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Dis Assoc Disord. 2006 Oct-Dec;20(4 Suppl 3):S152-69. PubMed.
External Citations
Further Reading
Papers
- Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer's disease: implications for prevention trials. Neuron. 2014 Nov 5;84(3):608-22. PubMed.
- Kryscio RJ, Abner EL, Cooper GE, Fardo DW, Jicha GA, Nelson PT, Smith CD, Van Eldik LJ, Wan L, Schmitt FA. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology. 2014 Oct 7;83(15):1359-65. Epub 2014 Sep 24 PubMed.
- Buckley RF, Ellis KA, Ames D, Rowe CC, Lautenschlager NT, Maruff P, Villemagne VL, Macaulay SL, Szoeke C, Martins RN, Masters CL, Savage G, Rainey-Smith SR, Rembach A, Saling MM, Australian Imaging Biomarkers and Lifestyle Study of Ageing (AIBL) Research Group. Phenomenological characterization of memory complaints in preclinical and prodromal Alzheimer's disease. Neuropsychology. 2015 Jul;29(4):571-81. Epub 2015 Feb 9 PubMed.
Primary Papers
- Amariglio RE, Donohue MC, Marshall GA, Rentz DM, Salmon DP, Ferris SH, Karantzoulis S, Aisen PS, Sperling RA, Alzheimer’s Disease Cooperative Study. Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer's Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol. 2015 Apr;72(4):446-54. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Metis Cognition Ltd.
There is without doubt a clear need for new measures of functional impairment. As the authors rightly point out, with the interest in AD focused largely on secondary prevention, appropriate and sensitive measures of functional activities are essential. Established measures such as the DAD and ADCS-ADL have rather shown their age with regard to content, and have been prone to ceiling effects in these early stage patients. The Clinical Dementia Rating (CDR) scale has become a popular alternative selection. Whilst the CDR can be a very useful staging instrument, many in the AD community, including the EMA, have expressed concerns about its utility as an outcome measure.
I think the appeal of the CDR has been that because it is a semi-structured interview, any comment made by a patient or their informant can be considered in scoring the patient’s levels of performance. These comments might reflect items not included on traditional ADL measures. Fixed item scales, which have focused on activities most commonly reported as being problematic, but not the entirety of function, are not as flexible, and that is challenging. Good examples of gaps with traditional measures would be buying things online, or organizing a tour-style vacation, etc. These have become relatively common activities and it is good to see that some elements of these issues have been incorporated in the CFI.
Personal performance on ADL items can also be idiosyncratic and especially influenced by cultural factors. I think by asking about change across a one year period this has been well-managed by the authors, because if the item is not appropriate then a ‘no change’ judgment can be selected. The challenge of having adopted this approach is whether the reporting of patients and informants can be considered accurate. This has long been an issue in the self-reporting capacities of patients’ who might exhibit anosognosia, possibly as an early consequence of AD. In this context it is interesting to see the authors state that the perceived change in function across 48 months by partners was more than twice that reported by patients.
Capturing levels of functional capacity has been a significant challenge for research in a number of CNS indications. An exciting development to have grown out of the ‘quantified self’ movement is the possibility of using mobile apps and ‘always-on’ gadgets to track and analyze people’s activities. I think this approach has great promise, but is very much in its infancy. In the meantime we will be largely reliant on patient and informant report measures. Recent innovations such as the CFI and the Amsterdam IADL scale are promising developments in an area with clear unmet need. Of course, one of the big questions is whether these scales can capture the effect of pharmaceutical and other interventions. I have found that sponsors have been unwilling to move away from scales like the DAD and CDR. However, it seems unlikely that these scales can capture interventional benefit, so arguably there is nothing to lose from trying newer, promising measures. It is worth mentioning that in contrast to the CDR, the CFI and AIADL can be administered by competent non-specialists. This is a clear benefit because the lack of appropriate CDR raters has been a major challenge in successfully running early stage AD clinical drug trials.
View all comments by John HarrisonUniversity of Cape Town
Tracking Early Decline with the CFI
The need for functional scales as outcomes for AD prevention trials has been identified. There are other scales available (ADL and iADL), but this one has the advantage of being able to be completed at home and returned by mail/email, by telephone, or face-to-face. For lengthy intervention trials in those at very early stages of cognitive decline, this is an obvious advantage over scales that only can be administered face-to-face. However, to provide reliable results, these sorts of questionnaires rely on the participants being honest, relatively well-educated, and not severely cognitively impaired.
The CFI does not attempt to replace cognitive outcome measures for trials, and in fact correlates well with the ADCS cognitive instrument (mADCS-PACC). Cognition will need to be assessed; however, interventions to improve cognition need to show efficacy for behavioral outcomes as well, to show that they are making a difference with activities of daily living.
The CFI was shown to predict cognitive decline to MCI and dementia over a four-year period. Its sensitivity to decline was only detectable after 12 months follow-up for both the partner report and when the self-report and partner-report scores were combined. Self-report only reached significance at 24 months. The self-report was reportedly more reliable in the earlier phase of decline (approximately between 24-36 months), while the partner report became more reliable when functional decline was more obvious (48 months). This is expected, because self-awareness of memory decline is better before decline is too marked, while a partner or informant may not be aware of early, subtle decline.
Overall, the differences in scores between those who progressed to MCI or mild dementia (CDR-G 0.5 or higher) are relatively small from a clinical perspective for the self-report (2.13 points in 48 months), although “self plus partner” reached a good degree of separation between the groups (7.5 points in 48 months). The problem is that not many trials of 48 months are easily affordable and compliance may fall and drop-outs increase. However, from a statistical analysis of trial efficacy, it looks as though trials of 24-36 months may also be long enough to show significant change.
The authors used modelling to test the scales as predictors of decline. This is not the same as testing improvement or maintenance of function due to intervention. However, the trajectories of decline that have been established will be useful as baselines for assessment of change due to interventions. The rate of change that can be expected with an intervention is therefore unknown, but would be expected to be less than the rate of decline, unless the intervention can completely restore the global function to normal (e.g. CDR-G = 0).
Another difficulty in a clinical trial is that the baseline cognitive/functional status of those randomized may not all be at the same level, especially in trials for those already experiencing memory complaints or who have MCI, thus change over time will be more variable.
The CFI does allow for inexpensive monitoring of reported functional ability, which will go a long way to making long-term AD prevention trials more feasible. The best results will be obtained with both self- and partner-report scores combined. Whether this scale will be sufficient, with other measures (cognitive, clinical etc.) only taken at baseline and trial end, remains to be seen. Alternatively, an intervention may be stopped after a period (for instance two years) with all measures done, and the CFI used in follow up to assess longer term benefits of the intervention.
View all comments by Celeste de JagerMake a Comment
To make a comment you must login or register.