Reinforcement, or Replacement? Stem Cell Strategies Divide to Conquer
Quick Links
Behind every successful neuron, there is a support crew of glia. Stem cell researchers are aiming to replace both types of cell in an effort to slow neurodegenerative disease. Two studies published October 13 in Stem Cell Reports described steps forward on either side of the coin. One, led by Mathew Blurton-Jones at the University of California, Irvine, reported that transplanted neural stem cells developed into glial cells that pumped out a trophic factor, which rescued motor and cognitive deficits in a mouse model of dementia with Lewy bodies (DLB). The other, led by Naihe Jing at the Chinese Academy of Sciences in Shanghai, took a more direct route, turning embryonic stem cells into cholinergic neurons that rescued memory problems in a mouse model of Alzheimer’s disease. A study published on the same day in Cell Stem Cell took yet another tack, reporting conversion of astrocytes into neurons—a shape-shifting process that perhaps one day can be induced in the brain rather than a dish. Which of these strategies is likely to pan out as a therapy? The answer will depend upon a slew of factors, including the nature and stage of the disease, but researchers agreed that attacking the problem from multiple angles is the way to go.
The defining characteristic of neurodegenerative disease is the death of neurons, and researchers have long searched for ways to either replace the fallen cells or bolster support for those that remain. Stem cell therapy offers opportunities to try both. Scientists have developed protocols to transform stem cells or induced pluripotent stem cells (iPSCs) into neurons of various persuasions, or into the glial cells that support them.
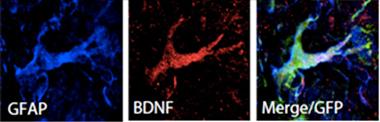
BDNF Bounty.
Transplanted neural stem cells differentiated into astrocytes (GFAP, blue) expressing the trophic factor BDNF (red). [Courtesy of Goldberg et al., 2015.]
Another approach is to skip the complexity of the stem cell altogether and directly deliver trophins such as brain-derived neurotrophic factor (BDNF) or nerve growth factor (NGF). The latter has a long history. In 2001, Mark Tuszynski and colleagues at the University of California, San Diego, delivered NGF to people with probable AD by injecting directly into the striatum either patient-derived fibroblasts engineered to pump out NGF, or adeno-associated virus (AAV) expressing NGF. Recently, the scientists reported postmortem results from 10 patients who died between one and 10 years later (see Tuszynski et al., 2015). The researchers observed neurons undergoing a growth spurt—putting out axonal projections and expressing key signaling molecules—in the areas near the injection. In the viral gene therapy recipients, both healthy neurons and degenerating cells riddled with tau tangles expressed NGF, indicating that even sickly neurons retain the capacity to produce trophic factors. While the results did not reveal whether the trophic support slowed the pace of AD in these patients, they suggest that when such therapy is delivered, neurons respond. The treatment, called CERE-110, ultimately failed to slow cognitive decline in AD patients in a Phase 2 trial. The program was terminated by Sangamo, the drug’s sponsor, earlier this year.
Viral delivery of BDNF has shown promise in rodent models of neurodegenerative disease as well as in aging primates (see Feb 2009 news; Nagahara et al., 2013). However, the treatment is still in its preclinical stages as researchers grapple with the challenge of efficiently delivering the trophin to specific regions in the brain (see Lynam et al., 2015).
Some researchers believe that the factors might work best when delivered by professionals (i.e., by glial cells that normally produce them). In Blurton-Jones’ paper, first author Natalie Goldberg and colleagues tried this in ASO mice, a model of DLB that overexpresses human α-synuclein. These animals are riddled with Lewy bodies and develop both motor and cognitive deficits. The researchers transplanted neural stem cells (NSCs) derived from normal mice directly into the striata of 12-month-old ASO mice. They found that the transplanted cells differentiated into astrocytes and oligodendrocytes, and six weeks after the injection had migrated throughout the striatum and even into the neighboring cortex and amygdala.
The transplanted cells restored the animals’ deteriorating motor function, as well as learning and memory. Animals harboring the cells performed at wild-type levels on the novel-object-recognition test, a cortical-dependent task that measures the ability to discriminate between new and familiar objects. The animals also performed at normal levels on a hippocampal-dependent memory task called the novel-place-recognition test, despite the fact that no transplanted cells were detected within the hippocampus. The researchers ultimately found that NSC transplantation boosted the activity of dopaminergic and glutaminergic neurons. While both types of neuron facilitated restoration of motor function, cognitive benefits primarily correlated with improved glutaminergic function.
The researchers ultimately found that both motor and cognitive improvements depended on BDNF being secreted by the transplanted cells, as NSCs lacking the BDNF gene did not restore these functions.
When the researchers delivered BDNF via AAV instead of stem cells, only the animals’ motor function improved, while cognition remained impaired. The researchers reported that NSC-delivered BDNF was of a 14kDa variety, while BDNF delivered by AAV also contained a 12kDa species. They speculated that this smaller form was less mature or possibly unphosphorylated, and may have failed to deliver the cognitive benefit derived from the more mature, NSC-delivered form of the protein. It could also be possible that the stem cells delivered BDNF in such a way that it reached glutaminergic neurons more efficiently. The retrograde transfer of BDNF along axons from the striatum into the cortex, for example, may have been responsible for rescue of cognitive function.
“These cells may have knowledge of their own about how to go to the right place,” commented Jeffrey Kordower of Rush University in Chicago, who was not involved in the study. Neither he nor Blurton-Jones, the senior author, suggested that the data ruled out viral-delivered BDNF as a potential treatment; however, both noted that the stem cells may have something unique to offer. This could be especially true for cognitive restoration, a function not normally associated with the striatum. “These types of studies are aimed at treating dementia symptoms in parkinsonian syndromes, which is a critical unmet need,” Kordower said.
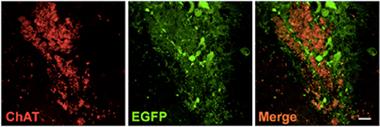
Cholinergic Conversion.
Transplanted neural progenitors (green, middle panel) differentiated into neurons expressing choline acetyl transferase (red, left). [Courtesy of Yue et al., Stem Cell Reports, 2015.]
Sending in trophic reinforcement may work while neurons are still alive, but could stem cells also replenish the ranks of dying neurons? In the second paper from Stem Cell Reports, co-first authors Wei Yue, Yuanyuan Li, Ting Zhang, and colleagues transplanted cholinergic neurons into the basal forebrain of a mouse model of AD. Cognitive decline in AD reflects a faltering cholinergic system, and drugs that boost production of acetylcholine are among the only ones to slightly soothe symptoms of the disease. The researchers sought to generate basal forebrain cholinergic neurons (BFCNs), which innervate the cortex and hippocampus, starting with embryonic stem cells (ESCs). Generating this neuronal subtype had proven difficult in the past, as researchers could never achieve cultures containing more 5 to 10 percent of these cells, Jing told Alzforum. However, they ultimately concocted a protocol that yielded roughly 40 percent cholinergic neurons.
The researchers transplanted the cells, while still in a progenitor stage, into the basal forebrain of two mouse models of AD—5xFAD and APP/PS1. They did so when each mouse strain had a heavy amyloid burden but no cognitive deficits yet. Two months after transfer, the researchers found that most of the transplanted cells had survived and differentiated into cholinergic neurons within the basal forebrain. The cells fired action potentials and formed neuronal projections that spread throughout the region, indicating that they had integrated into the existing neuronal circuitry. By this time, animals without the transplants had become slow at learning the location of a submerged platform, and mice harboring the BFCNs outperformed their untreated counterparts. The researchers concluded that the BFCNs relieved cognitive deficits by rescuing faltering cholinergic function in the AD mouse models. Unlike Alzheimer’s disease, these transgenic models do not feature extensive neuronal death before cognitive symptoms emerge. Interestingly, levels of BDNF also shot up in the brains of mice transplanted with the cells, but the researchers did not determine how much the trophic factor contributed to improved cognition, or which cells secreted it.
Blurton-Jones was impressed by the authors’ development of such a specific neuronal subtype and its integration into the cholinergic system. However, he noted that neuronal replacement in the context of AD, where many neuronal subtypes across the brain are degenerating, will raise many challenges. This will prove especially true in humans, he said, due to the sheer size of the brain, the number of neurons that need to be replaced, and the extensive projections that must form before the cells can alter cognition. While Blurton-Jones favors the trophic-support approach, he acknowledged that it would only work when initiated early in the disease process. Jing echoed this point, adding that by the time AD symptoms are apparent, extensive neurodegeneration is well underway. Importantly, neither treatment reduced the burden of amyloid burden in the AD mice or Lewy bodies in the ASO mice, an unsurprising finding to both researchers.
Yet another strategy to up the number of neurons in the brain is to convert glial cells into neurons. Reporting in Cell Stem Cell on October 13, researchers led by Gong Chen of Pennsylvania State University in University Park took a stab at this cellular transformation. Using a combination of small molecules, the researchers report successfully transforming human astrocytes into bona fide glutaminergic neurons that formed neuronal circuits in culture. When transplanted into mice, the cells lived for at least one month and formed connections with neighboring neurons, Chen and colleagues report.
Chen told Alzforum that ultimately he plans to induce this transformation within the brain. A problem he sees with stem cell transplantation techniques is that many of the transplanted cells die. Converting astrocytes into neurons in situ would circumvent that problem. However, many questions remain unanswered—for starters, how such a strategy could replace specific subtypes of neuron in the brain.—Jessica Shugart
References
Therapeutics Citations
News Citations
Research Models Citations
Paper Citations
- Tuszynski MH, Yang JH, Barba D, U HS, Bakay RA, Pay MM, Masliah E, Conner JM, Kobalka P, Roy S, Nagahara AH. Nerve Growth Factor Gene Therapy: Activation of Neuronal Responses in Alzheimer Disease. JAMA Neurol. 2015 Oct;72(10):1139-47. PubMed.
- Nagahara AH, Mateling M, Kovacs I, Wang L, Eggert S, Rockenstein E, Koo EH, Masliah E, Tuszynski MH. Early BDNF Treatment Ameliorates Cell Loss in the Entorhinal Cortex of APP Transgenic Mice. J Neurosci. 2013 Sep 25;33(39):15596-602. PubMed.
- Lynam DA, Shahriari D, Wolf KJ, Angart PA, Koffler J, Tuszynski MH, Chan C, Walton P, Sakamoto J. Brain derived neurotrophic factor release from layer-by-layer coated agarose nerve guidance scaffolds. Acta Biomater. 2015 May;18:128-31. Epub 2015 Feb 21 PubMed.
Further Reading
Primary Papers
- Goldberg NR, Caesar J, Park A, Sedgh S, Finogenov G, Masliah E, Davis J, Blurton-Jones M. Neural Stem Cells Rescue Cognitive and Motor Dysfunction in a Transgenic Model of Dementia with Lewy Bodies through a BDNF-Dependent Mechanism. Stem Cell Reports. 2015 Nov 10;5(5):791-804. Epub 2015 Oct 17 PubMed.
- Yue W, Li Y, Zhang T, Jiang M, Qian Y, Zhang M, Sheng N, Feng S, Tang K, Yu X, Shu Y, Yue C, Jing N. ESC-Derived Basal Forebrain Cholinergic Neurons Ameliorate the Cognitive Symptoms Associated with Alzheimer's Disease in Mouse Models. Stem Cell Reports. 2015 Nov 10;5(5):776-90. Epub 2015 Oct 17 PubMed.
- Zhang L, Yin JC, Yeh H, Ma NX, Lee G, Chen XA, Wang Y, Lin L, Chen L, Jin P, Wu GY, Chen G. Small Molecules Efficiently Reprogram Human Astroglial Cells into Functional Neurons. Cell Stem Cell. 2015 Oct 13; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.