Put a RING on It: Targeting Intracellular Tau Aggregates for Destruction
Quick Links
Immunotherapy can remove extracellular amyloid plaques from Alzheimer’s brain, but has so far failed to clear intracellular tau tangles. Now, two papers propose a solution: tagging them for destruction by the cell’s own machinery. The papers describe two different ways to hitch the catalytic RING domain of ubiquitin ligase to tangles. The strategies could potentially be used for other proteinopathies.
- A ubiquitin ligase conjugated to an anti-tau nanobody cleared tangles in mice.
- Replacing the nanobody with aggregation-prone tau achieved the same result.
- These strategies spare monomeric tau.
- Other aggregates could be cleared the same way.
As described in the August 30 Science, scientists led by William McEwan at the U.K. Dementia Research Institute, University of Cambridge, conjugated the RING domain to an anti-tau nanobody, an antibody fragment small enough to enter cells. As detailed in the September 10 Cell, researchers led by Leo James at the MRC Laboratory of Molecular Biology, also in Cambridge, attached the RING domain to an aggregation-prone isoform of tau. In both cases, the conjugate did its work, mopping up a third of pre-existing tangles in the brains of tauopathy mice within 10 days. The two labs collaborated on the studies.
McEwan noted that this way of targeting tangles is more potent and specific than anything tried so far. “We can selectively knock down aggregates, sparing monomers,” he told Alzforum.
“These are very promising and exciting strategies,” agreed Marc Diamond at University of Texas Southwestern Medical Center, Dallas. The major challenge for human therapy would be getting the agents into the brain in sufficient quantity, he noted (comment below).
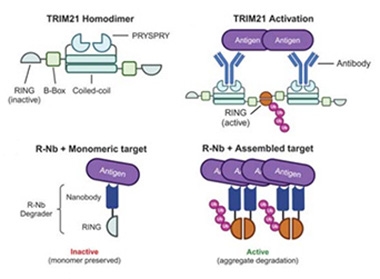
Aggregate Specificity. The ubiquitin ligase TRIM21 binds antibody-antigen complexes through its PRYSPRY domain (top left). Its RING domains are inactive until they dimerize (top right) and then begin adding ubiquitin (pink). A simplified construct consisting of a nanobody and RING domain (bottom left) likewise targets aggregated protein (bottom right). [Courtesy of Benn et al., Science/AAAS.]
Many efforts to curb tangles have employed anti-tau antibodies, which attempt to intercept tau assemblies as they pass between cells. So far, these approaches have failed to slow tangle spread (Jun 2021 news; Nov 2021 conference news; Jun 2022 news).
McEwan and James previously found that the E3 ubiquitin ligase TRIM21 assists antibodies to clear intracellular tau and prevent its seeding (Jan 2017 news). How does it do this? The ligase evolved to help the immune system fight microbial invaders. It carries a domain that recognizes antibody Fc stalks, and when multiple TRIM21 molecules bind the same antibody-antigen complex, its RING domains dimerize, forming a catalytically active ubiquitin ligase (Kiss et al., 2021). The ligase then adds ubiquitin chains to itself, marking it, and anything bound to it, for proteasomal degradation (image above). The scientists found that TRIM21 targeted tangles in the same way. The trick was to co-opt this system to directly target tangles, even when they formed intracellularly and with no antibodies in tow.
In the McEwan lab, joint first authors Jonathan Benn and Shi Cheng fused the TRIM21 RING domain to a nanobody specific for tau’s C-terminal end (image above). They tested it in HEK293 cells that overexpressed human P301S tau and had been seeded with small tau assemblies to spark aggregation. Fifteen hours after the cells expressed the construct, three-quarters of the tangles had disappeared. Nanobody-RING worked in a prevention paradigm as well, stopping tangles from forming when it was turned on before seeding.
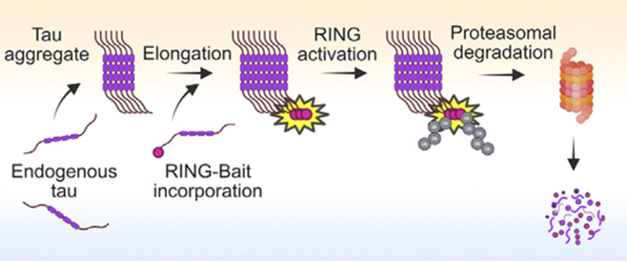
Baited Hook. A tau “bait” (purple) conjugated to a RING domain (red dot) incorporates into fibrils, activating (yellow) the ligase to add ubiquitin (gray) and trigger degradation. [Courtesy of Miller et al., Cell.]
In the James lab, joint first authors Lauren Miller and Guido Papa instead fused the RING domain directly to the C-terminus of full-length human P301S tau. In addition to the pathogenic mutation, this isoform contains four microtubule repeats and no extra N-terminal domains, making it particularly susceptible to clumping. Live cell imaging of fluorescently labeled tau-RING showed the construct being incorporated into tangles (diagram above). In HEK293 cells, tau-RING cleared 80 percent of pre-existing tangles within 72 hours, and prevented new ones.
Both approaches worked in mice as well. Benn and colleagues injected nanobody-RING into the frontal cortices of 6-month-old Tg2541 mice, which overexpress human P301S tau and have extensive tangles by then (Allen et al., 2002). This lowered tangles by a third in 10 days. This nanobody-RING performed similarly when it was expressed by an adenovirus engineered to enter the brain after being injected peripherally.

Stepping Pretty. Tauopathy mice treated with tau-RING (top) walked with a confident stride, while those treated with a catalytically inactive variant (bottom) shuffled along. [Courtesy of Miller et al., Cell.]
Meanwhile, in the James lab, Tg2541 mice injected in the tail vein with an adenovirus expressing tau-RING at 4 months of age had one-third as many tangles as did control mice two months later. Indeed, treated mice had about the same number of tangles as did 4-month-old Tg2541 controls, suggesting tau-RING expression had arrested disease progression. Supporting this, the treated mice maintained motor skills. Typically, as these mice age, they hunch over and shuffle along as they walk, but the tau-RING mice walked normally (image above).
Which RING method is better? McEwan noted that nanobody version works faster, probably because it can latch onto existing aggregates, whereas the tau chimera must be taken up as aggregates grow. Nanobody-RING is also more specific, since it will only recognize deposits with a particular shape. In in vitro experiments, the authors found that tau-RING could be incorporated into tau aggregates with different misfolded conformations, as long as the tau isoforms matched.
Einar Sigurdsson and colleagues at New York University believe nanobody-RING might be safer because of its higher specificity. For tau-RING, “the RING-domain could possibly be cleaved to some extent from the mutant tau, which could then become pathological,” they suggested (comment below).
Diamond had a different concern, noting that it would be difficult to get enough of these constructs into the human brain to achieve widespread tangle clearance. Adenoviral delivery works better in mouse brain because of its much smaller volume, he added.
McEwan agreed adenoviral technology is not quite good enough, though he noted that pharma companies are working on this, and he believes it will get there. In the interim, he and James are exploring other delivery methods, such as proteolysis-targeting chimeras, which use small, bivalent ligands to bring together an E3 ubiquitin ligase and a specific protein target, such as tau (March 2024 conference news).
On the basic science level, the scientists will use their constructs to examine what happens when different types of aggregate are selectively eliminated from mouse models. “There is this idea that some aggregates are protective. We can start to address which are the toxic ones,” McEwan told Alzforum.—Madolyn Bowman Rogers
References
News Citations
- Biogen Shelves Gosuranemab After Negative Alzheimer’s Trial
- More Tau Antibodies Bid Adieu; Semorinemab Keeps Foot in Door
- TAURIEL Phase 2 Data Published
- Antibodies Co-Opt Anti-Microbial Response to Clear Intraneuronal Tau
- Therapeutic Contenders Target Hard-to-Reach Pockets of Tau
Mutations Citations
Paper Citations
- Kiss L, Clift D, Renner N, Neuhaus D, James LC. RING domains act as both substrate and enzyme in a catalytic arrangement to drive self-anchored ubiquitination. Nat Commun. 2021 Feb 22;12(1):1220. PubMed.
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002 Nov 1;22(21):9340-51. PubMed.
Further Reading
News
- Tau Toggling Peptides: One Seeds Fibrils; the Other Dismantles Them
- p-Tau217 Immunotherapy Alleviates Tangle Pathology in Mice
- New Arrows Aimed at Tau: Single-Domain Antibody, Peptibody, Vaccine
- Two New Stabs at Vaccinating People Against Pathologic Tau
- Gosuranemab, Biogen’s Anti-Tau Immunotherapy, Does Not Fly for PSP
Primary Papers
- Benn J, Cheng S, Keeling S, Smith AE, Vaysburd MJ, Böken D, Miller LV, Katsinelos T, Franco C, Dupré E, Danis C, Landrieu I, Buée L, Klenerman D, James LC, McEwan WA. Aggregate-selective removal of pathological tau by clustering-activated degraders. Science. 2024 Aug 30;385(6712):1009-1016. Epub 2024 Aug 29 PubMed.
- Miller LV, Papa G, Vaysburd M, Cheng S, Sweeney PW, Smith A, Franco C, Katsinelos T, Huang M, Sanford SA, Benn J, Farnsworth J, Higginson K, Joyner H, McEwan WA, James LC. Co-opting templated aggregation to degrade pathogenic tau assemblies and improve motor function. Cell. 2024 Oct 17;187(21):5967-5980.e17. Epub 2024 Sep 13 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.