Proteomics Uncovers Potential Markers of Early Autosomal Dominant AD
Quick Links
Recent proteomic studies have offered glimpses into the biology of early Alzheimer’s disease. Two new preprints take this approach further, describing high-throughput surveys that turned up numerous new candidate biomarkers. In one, posted to medRxiv on January 13, scientists led by Carlos Cruchaga at Washington University in St. Louis reported 125 proteins in the CSF that distinguished people who had inherited a familial AD mutation from relatives who had not. Together, nine of these proteins differentiated carriers from noncarriers better than did established CSF Aβ and tau biomarkers. Many of these carriers, from the Dominantly Inherited Alzheimer Network cohort, were years or decades away from developing symptoms.
- In DIAN, largest CSF proteomic study to date finds 125 potential markers.
- A set of nine predicted the presence of FAD mutations better than did CSF Aβ or tau.
- Extracellular matrix protein SMOC1 could be the earliest biomarker.
- In sporadic AD, plasma analysis found completely different proteins.
“Studies such as this one … provide concrete evidence that AD-associated protein dysfunction, beyond Aβ and tau, occurs up to 30 years prior to symptom onset,” Eleanor Drummond at the University of Sydney wrote to Alzforum.
Meanwhile, researchers led by Asim Siddiqui at biotech Seer, Inc., in Redwood City, California, and Steven Arnold at Massachusetts General Hospital, Boston, focused on plasma proteins in people with sporadic AD. In a January 8 bioRxiv preprint, they reported levels of 138 correlated with disease, including eight that associated with cognitive decline. These had little overlap with the DIAN CSF set, indicating that biomarkers may be specific to plasma or CSF, or to disease type.

Candidate Biomarkers. A CSF proteome study of DIAN participants found 124 proteins that went up (red) and one that went down (blue) as symptoms approached. A42, p-tau, and tau shown as controls. [Courtesy of Shen et al., medRxiv.]
The DIAN study follows hundreds of families with inherited AD. The cohort includes people who carry presenilin and APP mutations, as well as their unaffected relatives. Previous studies in this cohort had identified proteome changes early in disease. For example, more than a decade ago, a study of 14 mutation carriers found 56 proteins up- or downregulated in the CSF, while a recent one in 22 mutation carriers found 66 (Jan 2012 news; Jul 2023 news). A larger study from Erik Johnson and colleagues at Emory University analyzed CSF samples from 286 mutation carriers and 184 noncarriers in DIAN, but examined only 59 proteins, linking 33 of them to the disease (Aug 2023 news). There had not yet been a large, unbiased proteomics study in this population.
For that, Cruchaga and colleagues assembled a large international team that included Johnson and colleagues. They also analyzed samples from the DIAN cohort, now comprising 291 carriers and 185 noncarriers. The participants’ mean age was 40, and about two-thirds of the FAD mutation carriers were asymptomatic. On average, participants were seven years shy of their estimated year of symptom onset (EYO). First author Yuanyuan Shen used the SomaScan commercial platform, which detects proteins that bind short oligonucleotides known as aptamers, to measure 6,163 proteins in a single CSF sample from each participant (Candia et al., 2017). By correlating protein abundance with EYO in these cross-sectional samples, Shen and colleagues estimated how protein levels changed as the volunteers aged.
This analysis turned up 125 proteins with divergent trajectories in mutation carriers compared with noncarriers. All but one of them, the synaptic protein NPTX2, ticked up in carriers over time. They included known AD proteins, such as NfL and neuregulin, but many had not been linked to AD previously. Overall, the set of 125 included extracellular matrix proteins SMOC2 and SLIT2, metabolic proteins such as PEBP1 and GPI, and cytoskeleton-related proteins such as STMN2 and PDLIM4. The findings jibed with previous DIAN studies. Of the 125 proteins, 24 had been examined in the earlier work, and all of these exhibited similar changes across studies.
Proteins that changed in coordinated fashion fell into several groups, or modules. In one, proteins started diverging from controls 13 years before EYO. This module consisted of 21 mostly neuronal proteins that were involved in NMDA synaptic signaling, cellular stress, and mitochondrial damage. In another module, proteins diverged 10 years before EYO. This set contained 24 neuronal and endothelial proteins involved in apoptosis, neuronal death, and immune response. In a third module, proteins changed from control levels about seven years before EYO. This module had 47 mostly microglial, astrocyte, and innate immune proteins.
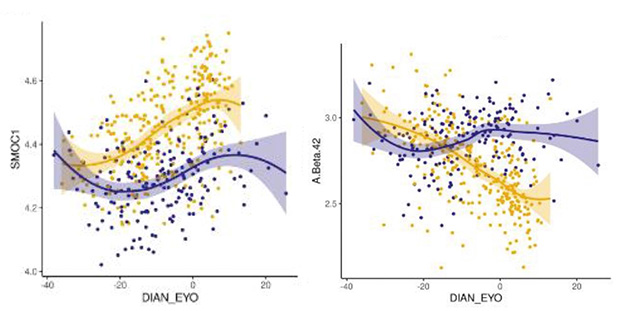
Earlier Marker. Extracellular matrix protein SMOC1 (left) begins to change 30 years prior to symptom onset in DIAN mutation carriers (gold) versus noncarriers (purple), beating Aβ42 (right) by 15 years. [Courtesy of Shen et al., medRxiv.]
Of particular interest was the ECM protein SMOC1, which rises 30 years before EYO and has turned up in several other proteome studies (Aug 2019 news; Feb 2022 news; Sep 2023 news). Betty Tijms and Lisa Vermunt at Amsterdam University Medical Center found this striking. “This indicates again that these SMOC proteins may be involved very early in the disease, and that we need to understand more about the processes related to this protein,” they wrote to Alzforum (comment below). Drummond agreed. “SMOC1 appears to be one of the most consistent fluid biomarkers of AD,” she wrote.
Using machine learning, Shen and colleagues identified nine proteins of the 125 that best identified people who carried an AD mutation. These were SMOC1, SMOC2, NPTX2, CAND1, PPP3CA, PRKCG, TMOD3, TREM1, and UBE2N. Together, the set nailed mutation status with an AUC of 0.89. This was better than the performance of p-tau181 and the p-tau/Aβ42 ratio, which both had an AUC of 0.80 in this cohort. However, p-tau and Aβ42 were better than the set of nine proteins at distinguishing symptomatic from presymptomatic carriers, consistent with the new set detecting very early changes, before symptoms appear.
“It is good to see replication of earlier findings from the Johnson study, and it is encouraging that both studies are detecting reproducible changes in CSF years before cognitive symptoms. This means the promise of early stage diagnostics could be realized,” noted Becky Carlyle at the University of Oxford, U.K.
What about biomarkers in plasma, which is easier to collect? These might be entirely different. Shen and colleagues found a much smaller set of 10 proteins that went up or down in ADAD plasma, with only SMOC1 in common with the CSF markers. Keenan Walker at the National Institute on Aging, Baltimore, noted that in sporadic AD, there is a large overlap between CSF and blood markers. “The findings suggest that, unlike late-life dementia syndromes, the proteomic signature for autosomal dominant AD is largely restricted to the CSF. By extension, this supports the idea that peripheral (non-CNS) factors play a larger role in determining risk for sporadic AD and related dementias emerging during late life,” he wrote to Alzforum (comment below).
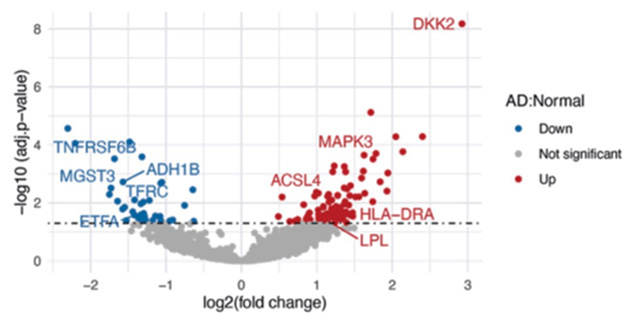
Plasma Proteins. Analysis of plasma from sporadic AD patients turned up a different set of elevated (red) and suppressed (blue) proteins. [Courtesy of Lacar et al., bioRxiv.]
Late-Onset AD Shows Up in Blood
In the second paper, Siddiqui and colleagues focused on the plasma signature of sporadic AD, and indeed found it was much more pronounced than in familial AD. Joint first authors Benjamin Lacar, Shadi Ferdosi, and Amir Alavi, all at Seer, used Proteograph technology, which detects proteins with nanoparticles, to measure more than 4,000 proteins in blood. Samples came from 1,006 participants in a longitudinal cohort study at the Massachusetts Alzheimer’s Disease Research Center. In this group, 379 people had AD, 387 other dementias, and 240 were cognitively healthy. The study included an average of two samples per participant.
Comparing AD to control samples, the authors identified 100 proteins that were more abundant in the former, and 38 that were less. Some of these, such as the kinase MAPK3, synaptic protein ACLS4, and apoptosis suppressor ADH1B, were known to have roles in AD. Others had not been directly tied to the disease before, but could help elucidate its mechanisms. For example, the most elevated protein, DKK2, inhibits the Wnt signaling pathway, which is known to be suppressed in AD. Metabolic protein MGST3 was down in AD patients, and has previously been associated with lower hippocampal size. Nonetheless, these plasma proteins did not perform as well as plasma p-tau181 in identifying AD patients, the authors noted.
The proteins might be more useful in predicting cognitive decline, with a set of eight associated with slippage on the CDR. Higher levels of BLVRB, CRISPLD2, CLNS1A, PRPS1, OXSR1, SELENBP1, SMYD5, and lower levels of GOLPH3, were linked to greater decline. “While some of the pathways and proteins identified are known to be involved with AD and related dementias, many are not and may point to novel biology,” the authors wrote. They believe the proteins predictive of decline could be used to aid treatment decisions in patients by flagging those most at risk.—Madolyn Bowman Rogers
References
News Citations
- Brain Proteins in Flux a Decade Before Familial AD Begins
- Proteomics Discerns Sporadic from Familial Alzheimer’s Disease
- Proteins in Biofluids Foreshadow Dementia by 30 Years
- Proteomics Uncovers Potential Markers, Subtypes of Alzheimer’s
- Proteomics Highlight Alzheimer’s Changes in Matrisome, MAPK Signaling
- CSF Proteomic Panel Better Predicts Decline Than Do Classic AD Biomarkers
Paper Citations
- Candia J, Cheung F, Kotliarov Y, Fantoni G, Sellers B, Griesman T, Huang J, Stuccio S, Zingone A, Ryan BM, Tsang JS, Biancotto A. Assessment of Variability in the SOMAscan Assay. Sci Rep. 2017 Oct 27;7(1):14248. PubMed.
Further Reading
Primary Papers
- Shen Y, Ali M, Liu M, Timsina J, Wang C, Do A, Western D, Gorijala P, Budde J, Liu H, Gordon B, Joseph-Mathurin N, Perrin RJ, McDade E, Morris JC, Llibre-Guerra JJ, Bateman RJ, Maschi D, Wyss-Coray T, Pastor P, Renton AE, Surace EI, Johnson EC, Alvarez I, Levin J, Ringman JM, Levey AI, Allegri RF, Seyfried N, Day GS, Wu Q, Fernandez MV, Ibanez L, Sung Y, Cruchaga C. Systematic proteomics in Autosomal dominant Alzheimer's disease reveals decades-early changes of CSF proteins in neuronal death, and immune pathways. 2024 Jan 13 10.1101/2024.01.12.24301242 (version 1) medRxiv.
- Lacar B, Ferdosi S, Alavi A, Stukalov A, Venkataraman GR, deGeus M, Dodge H, Wu C-Y, Kivisakk P, Das S, Guturu H, Hyman B, Batzoglou S, Arnold SE, Siddiqui A. Identification of Novel Biomarkers for Alzheimer's Disease and Related Dementias Using Unbiased Plasma Proteomics. 2024 Jan 08 10.1101/2024.01.05.574446 (version 1) bioRxiv.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.