PET Tracers For Non-Alzheimer’s Tauopathies Enter Clinical Testing
Quick Links
The current tau PET tracers bind best to the fibrils found in Alzheimer’s disease, and now, finally, comes progress on non-AD tauopathies. In the June 14 Nature Communications, researchers led by Neil Vasdev at the University of Toronto, in collaboration with Chester Mathis at the University of Pittsburgh and Samuel Svensson at the biotech Oxiant Discovery in Södertälje, Sweden, debuted a new tau tracer. In postmortem brain, OXD-2314 bound the four-repeat tau deposits characteristic of progressive supranuclear palsy and corticobasal degeneration, as well as 3R tau in Pick’s disease. In healthy rodents and nonhuman primates, radiolabeled OXD-2314 entered the brain and cleared quickly. The tracer is now in a Phase 1 study in healthy people.
- OXD-2314 binds 3R, 4R, and 3R/4R tau with high affinity in vitro.
- Phase 1 trial has begun.
- APN-1607 is starting a Phase 3 registration trial for 4R tauopathy PSP.
Rather than being derived from existing tracers, OXD-2314 was designed from scratch, with scientists generating a new chemical structure computationally predicted to bind 4R tau. “This is a new class of compound that can be explored. It’s a bench-to-bedside example of how we develop a new PET tracer,” Vasdev told Alzforum.
Further along in development, Aprinoia Therapeutics received Fast Track designation from the U.S. Food and Drug Administration to test APN-1607 in the most common 4R tauopathy, PSP. This FDA designation speeds up regulatory review, enabling products to come to market sooner. APN-1607, a pan-tau tracer, meaning it binds to all forms of tau fibrils, already has been tested in nearly 500 PSP patients. A worldwide Phase 3 study in PSP will begin enrolling later this year. If it succeeds, APN-1607 could become the first 4R tau tracer approved for clinical use. “We’re trying to bring it to patients as soon as possible,” Mark Shearman at Aprinoia told Alzforum, noting the urgent need for better ways to diagnose PSP. This disease is often mistaken for other disorders based on clinical symptoms.

In it Goes. In healthy rhesus macaques, OXD-2314 easily entered the brain, and washed out quickly. [Courtesy of Lindberg et al., 2024.]
Tau tracers already in research use, such as flortaucipir, were optimized to detect the mixed three- and four-repeat-containing fibrils found in AD brain. Some of them, for example Life Molecular Imaging’s PI-2620, also bind 4R tau, but the signal is typically weaker (May 2024 news). For this reason, Vasdev and colleagues set out to design a new compound that specifically bound 4R tau. Built around a pyridinyl-indole scaffold, their first candidate, OXD-2115, bound 4R fibrils in vitro but bounced off the blood-brain barrier (Lindberg et al., 2021).
Trying again, the authors synthesized and tested more than 150 analogues. First author Anton Lindberg identified OXD-2314 as the most promising one. It bound to homogenates from PSP brain with a dissociation constant of 2.4 nM, about twice as strongly as did OXD-2115, APN-1607, or PI-2620 in this assay. OXD-2314 bound equally well to homogenates from CBD brain, which contain 4R tau fibrils with a related, though distinct, fold from that in PSP.
Unexpectedly, OXD-2314 also bound 3R tau from Pick’s disease brain, and 3R/4R fibrils from AD homogenates, with a similar affinity. Computational modeling suggested it would bind the 3R/4R fibrils from chronic traumatic encephalopathy brain as well. AD and CTE folds resemble each other, and are more akin to the Pick’s fold than to the CBD and PSP folds (see Oct 2021 news for the tau fold family tree).
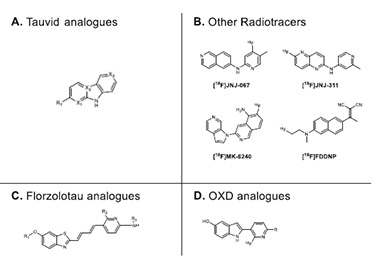
The Right Scaffold? The OXD-2314 pyridinyl-indole backbone (bottom right), distinguishes it from the carbazole of flortaucipir and PI-2620 (top left), the benzothiazole of PBB3 and APN-1607, aka florzolotau (bottom left), and the scaffolds of other PET tracers (top right). [Courtesy of Lindberg et al., 2024.]
The results suggest that, like APN-1607, OXD-2314 is a pan-tau tracer. The plan is to develop it for all the non-AD tauopathies, Vasdev told Alzforum. He does not know where OXD-2314 binds on tau fibrils, but competition assays showed the site is separate from where APN-1607, PI-2620, flortaucipir, and MK-6240 latch on.
Makoto Higuchi at the National Institutes for Quantum and Radiological Science and Technology in Chiba, Japan, said these data imply that OXD-2314 might bind two separate sites, with the lower-affinity binding accounting for perhaps a quarter of the total. Future work should identify this second site, which could potentially reflect binding to something other than tau, he suggested (comment below).
In mice, OXD-2314 injected into the blood readily entered the central nervous system, with a total brain-to-plasma ratio of 1.7. Values above 1 are desirable, Vasdev noted. The authors conjugated OXD-2314 to radiolabeled fluorine and tested the tracer in two rats, two rhesus macaque monkeys, and two baboons. Pharmacokinetics were favorable for a PET ligand, with the tracer peaking in the brain at two minutes, and half of it gone by around 20 minutes, as expected for healthy brain without tau aggregates (see image above).
In OXD-2314’s P1, the first two participants were scanned in June. Vasdev said the tracer entered the brain, produced a stable signal, and spawned no troublesome radiometabolites. “I’m delighted to say the tracer looks excellent in healthy controls,” he told Alzforum. The study is expected to conclude this summer, and by fall, the authors will test OXD-2314 in PSP patients. They hope to expand to CBD, Pick’s disease, and CTE.
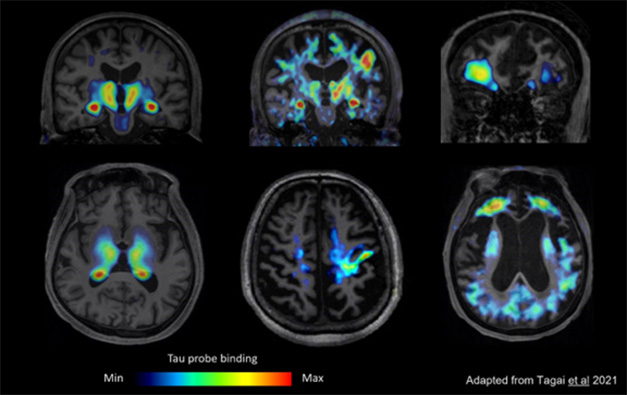
Each is Different. Tau tracer APN-1607 lights up the non-AD tauopathies PSP (left), CBD (middle), and Pick's disease (right) with distinct regional binding patterns. [Courtesy of Aprinoia.]
For its part, APN-1607 has been in research use for PSP and other disorders for seven years. More than 3,000 people have been scanned with it. Developed by Higuchi from the precursor PBB3, and originally known as PM-PBB3, the tracer now goes by the moniker florzolotau.
In PSP, its signal matches known patterns of fibril distribution, and correlates with clinical severity (Mar 2020 conference news). In addition, the PET signal matched subsequent autopsy findings from one PSP, one CBD, and one Pick’s patient (Tagai et al., 2020).
Based on these data, and the unmet need for 4R tracers, the FDA granted APN-1607 orphan drug designation for PSP in 2017. Fast Track was added May 8 2024 (see press release). The agency decided that a single Phase 3 trial, without formal autopsy confirmation, would be sufficient to demonstrate that this tracer would help diagnose PSP, Aprinoia’s Brad Navia told Alzforum. The pivotal trial will take place at several sites in Canada, Germany, Japan, Taiwan, the U.K., and the U.S., and will enroll 130 people with suspected PSP.
Beyond PSP, APN-1607 has also been used to scan about 70 people with CBD, and 140 people suspected of having tauopathy-driven frontotemporal lobar degeneration. Aprinoia will conduct Phase 3 trials in these conditions as well, Navia said. The company is also developing APN-1607 for AD, with a Phase 3 AD trial underway in China, and a Phase 2 study starting in the U.S.—Madolyn Bowman Rogers
References
News Citations
- Autopsies Confirm That PI-2620 Binds 4R Tau Deposits
- Flock of New Folds Fills in Tauopathy Family Tree
- Primary Tauopathies Get New PET Ligands
Paper Citations
- Lindberg A, Knight AC, Sohn D, Rakos L, Tong J, Radelet A, Mason NS, Stehouwer JS, Lopresti BJ, Klunk WE, Sandell J, Sandberg A, Hammarström P, Svensson S, Mathis CA, Vasdev N. Radiosynthesis, In Vitro and In Vivo Evaluation of [18F]CBD-2115 as a First-in-Class Radiotracer for Imaging 4R-Tauopathies. ACS Chem Neurosci. 2021 Feb 17;12(4):596-602. Epub 2021 Jan 26 PubMed.
- Tagai K, Ono M, Kubota M, Kitamura S, Takahata K, Seki C, Takado Y, Shinotoh H, Sano Y, Yamamoto Y, Matsuoka K, Takuwa H, Shimojo M, Takahashi M, Kawamura K, Kikuchi T, Okada M, Akiyama H, Suzuki H, Onaya M, Takeda T, Arai K, Arai N, Araki N, Saito Y, Trojanowski JQ, Lee VM, Mishra SK, Yamaguchi Y, Kimura Y, Ichise M, Tomita Y, Zhang MR, Suhara T, Shigeta M, Sahara N, Higuchi M, Shimada H. High-Contrast In Vivo Imaging of Tau Pathologies in Alzheimer's and Non-Alzheimer's Disease Tauopathies. Neuron. 2021 Jan 6;109(1):42-58.e8. Epub 2020 Oct 29 PubMed.
External Citations
Further Reading
Primary Papers
- Lindberg A, Murrell E, Tong J, Mason NS, Sohn D, Sandell J, Ström P, Stehouwer JS, Lopresti BJ, Viklund J, Svensson S, Mathis CA, Vasdev N. Ligand-based design of [18F]OXD-2314 for PET imaging in non-Alzheimer's disease tauopathies. Nat Commun. 2024 Jun 14;15(1):5109. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.