Parabiosis Illuminates Modifiable Pathways of Brain Aging
Quick Links
Aging and death are inescapable parts of life. But on a shorter timescale, certain aspects of aging may be delayed or even avoided altogether. A new study published March 9 in Nature Aging suggests as much by exposing age-related cellular pathways in the mouse brain that change when older animals are hooked up to the blood of a youngster. Using heterochronic parabiosis—the tethering of the circulatory system of young and old mice—followed by single cell transcriptomics, researchers led by Lee Rubin at Harvard University zeroed in on genes that changed in opposite directions in young versus old parabionts. They found age-related pathways involved in cell stress and injury, metabolism, inflammation, and proteostasis that were reversed by youthful systemic factors. Endothelial cells responded most robustly, suggesting a pivotal role for these cells in mediating aging—or anti-aging—effects in the brain.
- Scientists survey transcriptional changes in brains of old and young mice sharing blood vessels.
- Changes evoked hint at reversible aspects of brain aging.
- Endothelial cells responded most, revealing stress, injury and proteostasis pathways in aging.
More than a decade ago, researchers made the surprising discovery that molecules in the circulation could either speed up or slow down the aging process. By enjoining the circulatory systems of mice via parabiosis, or by transfusing animals with another’s blood, they demonstrated that factors within young blood rejuvenate neurogenesis, quell neuroinflammation, and boost metabolism and synaptic function in the brains of old mice, while blood from old mice could erode these functions in young’uns’ brains (Nov 2009 news; May 2014 news). Since then, Rubin and others have been hunting for specific blood factors that either speed or slow brain aging, with an eye toward developing therapeutics (Dec 2018 news; Ozek et al., 2018; Jun 2019 news).
Yet trawling young blood for rejuvenating compounds is but one side of the coin. It’s also important to identify the cellular processes in the brain that change with age; especially those that could be modifiable, Rubin said.
To try this, first author Methodios Ximerakis and colleagues used parabiosis to conjoin 3- to 4-month-old mice to 20- to 22-month-olds; as controls, they also joined together mice of the same age. A month later, they conducted single-cell transcriptomics on brain tissue isolated from the paired mice, as well as from unpaired mice of both ages. This combination of experimental conditions enabled the researchers to delineate age-related changes and to zero in on any that were altered by the heterochronic blood.
They identified 31 major cell types from among 105,000 cells included in the analysis. Overall, parabiosis did not change the numbers of any particular cell type in either young or old animals.
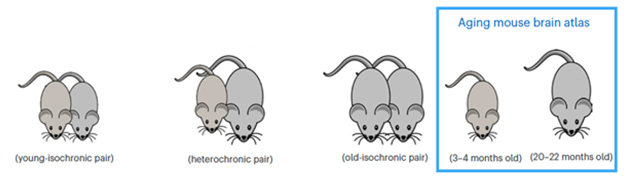
Pairing Up. Heterochronic parabiosis involves the joining of the blood supply of young and old mice. Controls included isochronic pairs of the same age, as well as unpaired mice of different ages. [Courtesy of Ximerakis et al., Nature Aging, 2023]
Ximerakis et al. next looked for differentially expressed genes to identify signatures associated with “rejuvenation” or “aging acceleration” in old and young parabionts, respectively. They found 442 genes that changed expression under rejuvenating conditions, and 155 that changed under accelerated aging conditions. These genes were expressed in oligodendrocytes, astrocytes, neurons, microglia, and endothelial cells. Among these genes, 41 changed expression in opposite directions in rejuvenating versus faster aging scenarios.
Perhaps unexpectedly, it was the humble endothelial cell that exhibited the highest number of genes, 17, that changed expression bidirectionally. Most of these fell quiet in old but rejuvenated parabionts, while their expression roared in old, unpaired mice or in young mice fused to old.
These aging-related genes encoded proteins involved in stress responses, such as heat-shock proteins, as well as proteins mediating vascular injury, inflammation, senescence, metabolism, and proteostasis. “Collectively, these data suggested that heterochronic parabiosis changes the metabolic profile, improves proteostatic machinery, and reduces aging-associated apoptosis or senescence to improve endothelial cell function,” the authors wrote. The dataset is publicly available (https://singlecell.broadinstitute.org/single_cell/study/SCP2011/aging-mouse-brain-parabiosis).
The findings highlight the role of endothelial cells in brain aging, and suggest that these cells may be among the most malleable when it comes to responding to therapeutics, Rubin said. While endothelial cells are able to multiply in response to injury, they are also extremely long-lived. This suggests that, like neurons, they cope with mounting burdens of age, such as eroding endolysosomal function and proteostasis.
Rubin views this research through a translational lens. “We want to use the data to guide development of effective therapeutics,” he said. He believes this could be helpful not only for neurodegenerative diseases such as AD, but also for conditions commonly considered a normal part of the aging process, such as a certain slowing and dulling of cognition. Muscle loss is already considered a modifiable aspect of aging, Rubin noted. “I don’t see why cognitive loss should be any different.”—Jessica Shugart
References
News Citations
- Chicago: The Vampire Principle—Young Blood Rejuvenates Aging Brain?
- In Revival of Parabiosis, Young Blood Rejuvenates Aging Microglia, Cognition
- How Immune Cells From Blood Beget Aging in Brain
- Two Proteins in Young Blood Give Synapses a SPARC
Paper Citations
- Ozek C, Krolewski RC, Buchanan SM, Rubin LL. Growth Differentiation Factor 11 treatment leads to neuronal and vascular improvements in the hippocampus of aged mice. Sci Rep. 2018 Nov 23;8(1):17293. PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Ximerakis M, Holton KM, Giadone RM, Ozek C, Saxena M, Santiago S, Adiconis X, Dionne D, Nguyen L, Shah KM, Goldstein JM, Gasperini C, Gampierakis IA, Lipnick SL, Simmons SK, Buchanan SM, Wagers AJ, Regev A, Levin JZ, Rubin LL. Heterochronic parabiosis reprograms the mouse brain transcriptome by shifting aging signatures in multiple cell types. Nat Aging, March 9, 2023
Annotate
To make an annotation you must Login or Register.

Comments
Icahn School of Medicine at Mount Sinai
This exciting work from Lee Rubin’s group is a fantastic resource for the expanding field of blood-CNS aging research. Ximerakis, Holton, and colleagues set out to characterize how, at single-cell resolution, the aged or young mouse brain responds to sharing of either young or aged blood, respectively. Particularly striking changes are present in brain endothelial cells, with many of these changes altered in both “rejuvenation” and “aging acceleration” contexts.
This work dovetails nicely with the group’s prior work showing that exposure to young blood through heterochronic parabiosis rejuvenates the aged brain vasculature (Katsimpardi et al., 2014). Based on their new analyses, endothelial cells appear to be among the most responsive, which might be expected given their proximity to the circulating signals that likely mediate blood-brain communication, as highlighted in recent work (Yang et al., 2020).
Interestingly, exposure to young blood in aged mice appears to upregulate pathways associated with mitochondrial function, which are downregulated in aged mice compared to young mice. This finding is particularly convincing, given that a previous whole-body, single-cell RNA-Seq dataset in heterochronic parabionts found similar changes in mitochondrial activity-associated pathways in various tissues (Pálovics et al., 2022).
Intriguingly, the study also implicates parabiosis in reprogramming of transcriptional signatures in the brain, raising the possibility of identifying epigenetic mediators in future work. Beyond endothelial cells, the new dataset also points to oligodendrocytes and other glia as being particularly responsive to these factors. These new insights and other intersections with the growing number of atlases of aging interventions should surely shed light on the molecular pathways governing blood-CNS communication.
Taken together, these results add to the growing body of evidence revealing that diverse classes of CNS cells respond to circulating factors. Many groups, including the Rubin group, have put forth intriguing candidates that mediate some of these rejuvenation or age acceleration effects (Castellano et al., 2017; Katsimpardi et al., 2014; Khrimian et al., 2017; Smith et al., 2015; Villeda et al., 2011). This work argues there is much to be learned from understanding the cellular programs altered by exposure to blood factors, programs that represent plausible targets for therapies for age-associated disorders.
Future work can characterize the extent to which these programs, identified in the context of the parabiosis model, are recapitulated in other, clinically amenable models, including plasma transfer or treatments with proteins such as GDF11 or others, and the extent to which altered cellular programs exhibit brain region-specific sensitivity to blood-sharing. Ultimately, how these programs can be exploited therapeutically to target age-related brain disorders will be a key direction.
References:
Castellano JM, Mosher KI, Abbey RJ, McBride AA, James ML, Berdnik D, Shen JC, Zou B, Xie XS, Tingle M, Hinkson IV, Angst MS, Wyss-Coray T. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature. 2017 Apr 19; PubMed.
Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014 May 9;344(6184):630-4. Epub 2014 May 5 PubMed.
Khrimian L, Obri A, Ramos-Brossier M, Rousseaud A, Moriceau S, Nicot AS, Mera P, Kosmidis S, Karnavas T, Saudou F, Gao XB, Oury F, Kandel E, Karsenty G. Gpr158 mediates osteocalcin's regulation of cognition. J Exp Med. 2017 Oct 2;214(10):2859-2873. Epub 2017 Aug 29 PubMed.
Pálovics R, Keller A, Schaum N, Tan W, Fehlmann T, Borja M, Kern F, Bonanno L, Calcuttawala K, Webber J, McGeever A, Tabula Muris Consortium, Luo J, Pisco AO, Karkanias J, Neff NF, Darmanis S, Quake SR, Wyss-Coray T. Molecular hallmarks of heterochronic parabiosis at single-cell resolution. Nature. 2022 Mar;603(7900):309-314. Epub 2022 Mar 2 PubMed.
Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, Wheatley EG, Bouchard J, Eggel A, Narasimha R, Grant JL, Luo J, Wyss-Coray T, Villeda SA. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015 Aug;21(8):932-7. Epub 2015 Jul 6 PubMed.
Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Després S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011 Sep 1;477(7362):90-4. PubMed.
Yang AC, Stevens MY, Chen MB, Lee DP, Stähli D, Gate D, Contrepois K, Chen W, Iram T, Zhang L, Vest RT, Chaney A, Lehallier B, Olsson N, du Bois H, Hsieh R, Cropper HC, Berdnik D, Li L, Wang EY, Traber GM, Bertozzi CR, Luo J, Snyder MP, Elias JE, Quake SR, James ML, Wyss-Coray T. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature. 2020 Jul 1; PubMed.
Make a Comment
To make a comment you must login or register.