Nix Tryptophan Metabolite, Temper Alzheimer’s?
Quick Links
Could one tiny little amino acid derivative make Alzheimer’s disease a whole lot worse? In the August 25 Science, researchers led by Katrin Andreasson, Stanford University School of Medicine, report that, in response to oligomers of Aβ or tau, astrocytes produce copious amounts of the tryptophan metabolite kynurenine, which scuppers glycolysis and lactate production. Starved of lactate, neurons crater and synaptic plasticity withers.
- Kynurenine, a tryptophan metabolite, slows astrocyte glycolysis in AD models.
- With no lactate from astrocytes, neurons falter.
- Blocking kynurenine production rescued cognition in models of amyloidosis and tauopathy.
The good news? All this might be prevented by way of small-molecule inhibitors of indoleamine-2,3-dioxygenase 1, which converts tryptophan to kynurenine. IDO1 inhibitors have been developed as adjunct cancer drugs because some tumors evade the immune system when they spew out the tryptophan derivative—it also happens to suppress monocyte and T cell responses. Whether these drugs could be repurposed for AD and other neurodegenerative diseases, such as tauopathies, needs to be investigated, said Andreasson.
“This study … is outstanding in its rigor and significance for both Alzheimer’s disease and other neurodegenerative conditions,” wrote Bruce Brew, University of New South Wales, Sydney, to Alzforum (comment below). “It provides a new mechanistic insight into the cause of AD, thereby facilitating potential new therapies.”
Qing Yang, Fudan University, Shanghai, thought it significant for examining the unexplored role of IDO1 in regulating metabolism in AD. “This pivotal report will pave the way for future studies on IDO1 and metabolic disruption in neurodegenerative diseases,” she wrote (comment below).
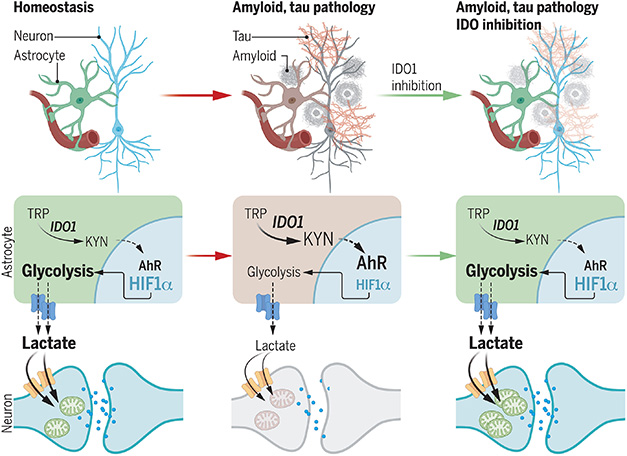
Save the Lactate Shunt. Astrocytes normally feed lactate to neurons (left). This dries up when Aβ or tau oligomers activate IDO1, causing a flood of kynurenine that suppresses astrocyte glycolysis (center). Blocking IDO1 restores normal metabolism (right). [Courtesy of Minhas et al., 2024.]
Kynurenine got its name from dog urine, the fluid in which it was first found. It exerts multiple biological effects. In the 1980s, scientists found it metabolizes to compounds that either cause excitotoxicity, such as quinolinic acid, or protect against it, such as kynurenic acid (Foster et al., 1983; Foster et al., 1984). Later, Michael Platten’s lab at the University Hospital of Heidelberg, Germany, reported that it activates the aryl hydrocarbon receptor (AhR), a transcriptional regulator that can be triggered by toxic chemicals, such as dioxin, but had no known endogenous ligand at the time (Opitz et al., 2011). Later, Yang reported that Aβ toxicity depends on the kynurenine-AhR pathway, while others found that, in plasma, the tryptophan metabolite correlated with markers of neurodegeneration in people with early AD (Duan et al., 2020; Chatterjee et al., 2019). In the Framingham Health Study, plasma kynurenine pathway markers also hinted at future dementia (Chouraki et al., 2017). For such a small molecule, kynurenine seems to wield a lot of power.
Andreasson was curious about kynurenine’s role in immune responses. First author Paras Minhas and colleagues had found that kynurenine drives production of NAD+ in macrophages, and that without this pathway, effector function and phagocytosis petered out (Minhas et al., 2019). To learn whether this is relevant for AD, Minhas crossed IDO1-negative mice with an APP/PS1 model of amyloidosis. “We fully expected these crosses to be worse. Instead, we were shocked to see the complete opposite,” Andreasson told Alzforum. The mice lacking IDO1 seemed normal.
What was going on? To find out, the authors examined astrocytes. In the brain, neurons make very little IDO1, but the glia make a lot, and different pathologies can induce it, said Andreasson. Sure enough, when Minhas probed mouse primary astrocytes or iPSC-derived human astrocytes with oligomers of Aβ or tau, the cells made more IDO1 and they cranked out more kynurenine. “It was then a step-by-step approach to find out how this affected the mice,” said Andreasson.
Minhas found that the kynurenine bound to AhR. This, in turn, bound the AhR nuclear transporter ARNT. The liaison left another ARNT partner, hypoxia inducible factor 1a, out in the cold. In astrocytes, this transcription factor drives glycolysis and production of lactate. The data suggested that by triggering IDO1 and driving up kynurenine, toxic forms of Aβ and tau might block the astrocyte lactate shunt, an essential source of energy for neurons.
Turning to animal models, that’s exactly what the authors found. APP/PS1 and 5xFAD mice had more kynurenine, weaker glucose metabolism, and less lactate in their hippocampus than did wild-type mice.
Lactate levels reverted to normal in mice treated with the IDO1 inhibitor PF068 (image below). Furthermore, this compound restored spatial memory, as tested in the Barnes water maze and novel object recognition tests, in 5- to 6-month-old 5xFAD mice and in 10- to 12-month-old APP/PS1 mice. This inhibitor also rescued spatial memory in the PS19 model of tauopathy.

Sweeter Without IDO1. Analysis of metabolites in the hippocampi of 5xFAD mice (left) shows that blocking IDO1 with PF068 boosts rescues glycolysis and the tricarboxylic acid cycle (right). [Courtesy of Minhas et al., 2024.]
Direct evidence that restoring the lactate shunt explained this rescue came from hippocampal slices. PF068 restored long-term potentiation in slices from all three mouse models, but not if monocarboxylate transporters were also blocked. These transporters shunt lactate from astrocytes to neurons.
All told, the data suggest that in AD and tauopathy models, astrocyte production of IDO1 weakens the cells’ support of nearby neurons. Hints that this might be going on in AD came from postmortem brain samples and human iPSC lines. In middle frontal gyrus tissue, kynurenine levels, but not tryptophan levels, ticked up with increasing Braak stage. IPSC-derived astrocytes from people with late-onset AD had more kynurenine and reduced glycolysis compared to cells from normal donors, while induced neurons in co-culture took up little lactate. In both cell types, PF068 restored normal metabolism.
Erik Johnson, Emory University, Atlanta, thought the study was impressive. “The authors provide compelling evidence that normal glucose metabolism in astrocytes is essential for proper neuronal function in AD model systems,” he wrote (comment below). “Interestingly, the authors did not observe a change in GFAP levels with IDO1 inhibition, suggesting that astrocytosis, as measured by this marker, can be decoupled from astrocytic glycolysis.” He thinks it would be interesting to see if IDO1 inhibition affects the FDG-PET signal, which decreases in AD brain.
Could IDO1 inhibitors become AD drugs? Several have been tested for cancer but information on them is limited, noted Andreasson. Pfizer tested PF068, also called EOS200271, in a Phase 1 trial for malignant gliomas, but terminated the program. Eli Lilly terminated a program testing their anti IDO1 agent LY3381916 for solid tumors, as did Bristol-Myers Squibb for their IDO1 inhibitor BMS98620 in combination with nivolumab. According to ClinicalTrials.gov, this was due to toxicity in people with stage II-IV squamous cell cancer of the head. Incyte tested INCB024360, aka Epacadostat, in combination with Merck’s Keytruda, for melanoma in Phase 3 but it missed its primary endpoint.
Brew cautioned that some of these compounds cross-react with AhR, which may defeat the purpose of blocking IDO1. He also noted that since the kynurenine pathway is one of only three mechanisms for NAD+ production, modulation rather than inhibition might be the better strategy.
Andreasson told Alzforum that the Pfizer compound is the only one that clearly penetrates the brain, a requirement for testing in AD. She would like to work with clinicians to evaluate such compounds for AD but said she’s hit a wall with pharmaceutical companies. “We are a bit in the dark on most of these compounds and we’d need a lot more information before we could consider potential trials,” she said.—Tom Fagan
References
Research Models Citations
Paper Citations
- Foster AC, Collins JF, Schwarcz R. On the excitotoxic properties of quinolinic acid, 2,3-piperidine dicarboxylic acids and structurally related compounds. Neuropharmacology. 1983 Dec;22(12A):1331-42. PubMed.
- Foster AC, Vezzani A, French ED, Schwarcz R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett. 1984 Aug 10;48(3):273-8. PubMed.
- Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011 Oct 5;478(7368):197-203. PubMed.
- Duan Z, Zhang S, Liang H, Xing Z, Guo L, Shi L, Du L, Kuang C, Takikawa O, Yang Q. Amyloid β neurotoxicity is IDO1-Kyn-AhR dependent and blocked by IDO1 inhibitor. Signal Transduct Target Ther. 2020 Jun 12;5(1):96. PubMed.
- Chatterjee P, Zetterberg H, Goozee K, Lim CK, Jacobs KR, Ashton NJ, Hye A, Pedrini S, Sohrabi HR, Shah T, Asih PR, Dave P, Shen K, Taddei K, Lovejoy DB, Guillemin GJ, Blennow K, Martins RN. Plasma neurofilament light chain and amyloid-β are associated with the kynurenine pathway metabolites in preclinical Alzheimer's disease. J Neuroinflammation. 2019 Oct 10;16(1):186. PubMed.
- Chouraki V, Preis SR, Yang Q, Beiser A, Li S, Larson MG, Weinstein G, Wang TJ, Gerszten RE, Vasan RS, Seshadri S. Association of amine biomarkers with incident dementia and Alzheimer's disease in the Framingham Study. Alzheimers Dement. 2017 Jun 8; PubMed.
- Minhas PS, Liu L, Moon PK, Joshi AU, Dove C, Mhatre S, Contrepois K, Wang Q, Lee BA, Coronado M, Bernstein D, Snyder MP, Migaud M, Majeti R, Mochly-Rosen D, Rabinowitz JD, Andreasson KI. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat Immunol. 2019 Jan;20(1):50-63. Epub 2018 Nov 26 PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Minhas PS, Jones JR, Latif-Hernandez A, Sugiura Y, Durairaj AS, Wang Q, Mhatre SD, Uenaka T, Crapser J, Conley T, Ennerfelt H, Jung YJ, Liu L, Prasad P, Jenkins BC, Ay YA, Matrongolo M, Goodman R, Newmeyer T, Heard K, Kang A, Wilson EN, Yang T, Ullian EM, Serrano GE, Beach TG, Wernig M, Rabinowitz JD, Suematsu M, Longo FM, McReynolds MR, Gage FH, Andreasson KI. Restoring hippocampal glucose metabolism rescues cognition across Alzheimer's disease pathologies. Science. 2024 Aug 23;385(6711):eabm6131. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.