New Type of Toxic Tau? Acetylated Form Correlates With Memory Defects
Quick Links
Tau researchers are aware that their favorite protein is subject to myriad modifications beyond phosphorylation, but they don’t quite know which ones matter most to disease. In the September 21 Nature Medicine, researchers led by Li Gan of the Gladstone Institute of Neurological Disease, San Francisco, strengthen the case for the acetylation of tau contributing to Alzheimer’s and other tauopathies. They report that an excess of tau acetylated at a particular site correlated with brain atrophy, as well as memory and behavior problems, in tauopathy model mice. Conversely, blocking this acetylation preserved cognition and led to improvements in pathology. Some of this work was previously presented at a conference in San Francisco jointly sponsored by the Gladstone Institute and the German Center for Neurodegenerative Diseases, Bonn (see May 2013 conference news).
Commentators praised the study for drawing attention to the role of other post-translational modifications. “This elegant study goes all the way from identifying a particular modification to giving an idea of its pathophysiological significance. It strongly supports the notion that acetylation is a dominant modification that regulates tau function,” noted Todd Cohen at the University of North Carolina, Chapel Hill.
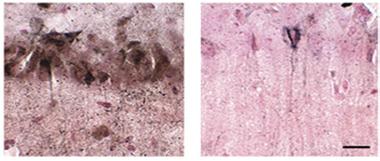
No Acetylation, No Tangles?
In tauopathy mice treated with the acetylation blocker salsalate (right), few neurofibrillary tangles (brown) form compared to controls (left). [Courtesy of Min et al., Nature Medicine.]
In the last five years, evidence for the importance of this form of tau has been growing. Gan first reported that acetylation slowed tau degradation, leading to the accumulation of pathogenic forms of the protein (see Sep 2010 news). Meanwhile Cohen, then working with Virginia Lee at the University of the Pennsylvania School of Medicine in Philadelphia, reported the presence of acetylated tau in several human tauopathies, but not healthy tissue, and implicated it in promoting tau aggregation (see Mar 2012 news). It was unclear, however, which acetylation sites might drive pathology.
To investigate this, joint first authors Sang-Won Min and Xu Chen analyzed samples from human postmortem brains by mass spectrometry and immunohistochemistry. They found that 21 Alzheimer’s brains, but not seven healthy ones, contained tau acetylated at lysine residue 174. This form appeared at Braak stage I, with soluble levels remaining steady through later stages. The authors did not measure insoluble tau, which might continue to accumulate as disease progresses, Gan noted. The same acetylated form appeared in PS19 mice, which express mutant human P301S tau and develop neurofibrillary tangles and cognitive deficits. The authors did not examine any human brains with pure tauopathies.
What might this acetylated tau do in cells? The authors made a mutant form of tau that mimicked the acetylated state by replacing lysine with glutamine. In cell culture, this mimic, K174Q, slowed tau degradation just as the acetylated form did. The authors next overexpressed K174Q or wild-type tau in young wild-type mice by injecting viral vectors into the hippocampus. Three months later, mice that expressed the acetylation mimic had lost about 40 percent of their hippocampal volume, compared to 20 percent in those with too much wild-type tau. Mice with K174Q tau scurried around more in the open-field assay, suggesting perhaps a hippocampal defect, and performed poorly in the Y and Morris water mazes. In contrast, wild-type tau over-expressers displayed no such problems. The findings imply a toxic function for K174Q independent of its effect on tau accumulation, Gan said.
The authors next asked if targeting acetylation would reverse this toxicity. They focused on the acetyltransferase p300, which previously had been shown to be one of two main enzymes responsible for adding acetyl groups to tau (see Cohen et al., 2011). The authors inhibited p300 in middle-aged PS19 mice by feeding the animals salsalate, a nonsteroidal anti-inflammatory drug (NSAID) used to treat rheumatoid arthritis. Two to three months of treatment preserved hippocampal volume and slashed the number of neurofibrillary tangles by up to two-thirds (see image above). Moreover, treated animals maintained their memories better than their untreated littermates.
Was the protection by salsalate due to lowering acetylated tau, however? The drug also works in other ways, and p300 acetylates numerous other targets, including histones that affect transcription. To explore this, the authors injected wild-type mice with a vector expressing K174Q, waited two months, then fed half the animals salsalate for another month. Treated and untreated animals resembled each other, with comparable hippocampal atrophy and tau levels. The lack of benefit from salsalate in mice expressing the acetylation mimic suggested the drug has to prevent tau acetylation in order to protect the brain, Gan noted.
The results imply that inhibiting p300 could be a therapeutic strategy, Gan told Alzforum. She noted that this enzyme has been found to be overactive in AD brains, as well as in PS19 mice (see Aubry et al., 2015). Because salsalate is an approved drug with a good safety profile, it might be worth testing in AD patients, she suggested. The dose used in this study equates to about 1,350 mg/day in people, lower than the typical dose of 3,000 mg/day taken for arthritis. Adam Boxer at the University of San Francisco is conducting a small pilot study of salsalate in people with progressive supranuclear palsy, a pure tauopathy.
Aspirin (acetylsalicylate) is chemically similar to salsalate (salicylsalicylic acid) and is metabolized to the same form (salicylate). Alas, it is unclear if it would have the same benefits as the NSAID, Gan said. In cell cultures, aspirin had opposite effects, rapidly losing its acetyl group and boosting tau acetylation. However, in people aspirin might be metabolized in the bloodstream, so that only salicylate enters the brain, Gan noted. She added that other NSAIDs are unlikely to have the same effects on p300 as salsalate, and therefore probably are not candidates to dampen acetylation. Gan is searching for more potent, brain-penetrant inhibitors of p300.
Many questions remain about the role of the K174 acetylation site in human disease. Gan is studying how the acetylated form might affect tau localization and neuronal plasticity. She noted that this acetylation site did not crop up in a recent survey of tau modifications by Lennart Mucke and colleagues at Gladstone (see Jul 2015 news). This might be because that survey looked at mouse tau rather than human, and K174 lies in a poorly conserved region, Gan suggested. Alternatively, it could be because acetylated K174 tau represents a pathogenic form not present in the mice used in that study, which modeled amyloidosis and accumulated little tau pathology.
Moreover, while acetylation at residue K174 appears sufficient for toxicity in this study, Gan stressed that there are likely to be additional important acetylation sites, as well as diverse toxic forms of tau. Other researchers agreed. Indeed, a recent study by scientists at Merck, this one of rTg4510 tauopathy model mice, found that pathological, aggregated tau accumulated numerous modifications, including phosphorylation, acetylation, ubiquitination, and nitration (see Song et al., 2015). The Merck scientists argued for therapeutic targeting of multiple modifications that are found to be present in pools of pathological tau. Cohen also noted that combinations of modifications might be important. Tara Spires-Jones at the University of Edinburgh wrote to Alzforum, “Similar effects on reducing pathology and improving cognition have been seen in mice when targeting different phospho-epitopes of tau and tau oligomers … It may take multiple approaches to effectively prevent tau toxicity in humans.” (See full comment below.)—Madolyn Bowman Rogers
References
News Citations
- Therapeutic Approaches Target Deubiquitinase, Protein Turnover
- Tau Timing: New Findings on Disease Progression, Clearance
- Can a Little Sugar Keep Tau From Souring Neurons?
- Inventory of Tau Modifications Hints at Undiscovered Functions
Research Models Citations
Paper Citations
- Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, Lee VM. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. 2011;2:252. PubMed.
- Aubry S, Shin W, Crary JF, Lefort R, Qureshi YH, Lefebvre C, Califano A, Shelanski ML. Assembly and interrogation of Alzheimer's disease genetic networks reveal novel regulators of progression. PLoS One. 2015;10(3):e0120352. Epub 2015 Mar 17 PubMed.
- Song L, Lu SX, Ouyang X, Melchor J, Lee J, Terracina G, Wang X, Hyde L, Hess JF, Parker EM, Zhang L. Analysis of tau post-translational modifications in rTg4510 mice, a model of tau pathology. Mol Neurodegener. 2015 Mar 26;10:14. PubMed.
External Citations
Further Reading
Primary Papers
- Min SW, Chen X, Tracy TE, Li Y, Zhou Y, Wang C, Shirakawa K, Minami SS, Defensor E, Mok SA, Sohn PD, Schilling B, Cong X, Ellerby L, Gibson BW, Johnson J, Krogan N, Shamloo M, Gestwicki J, Masliah E, Verdin E, Gan L. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med. 2015 Oct;21(10):1154-62. Epub 2015 Sep 21 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Edinburgh
Large amounts of evidence support the idea that abnormal levels of soluble tau are toxic and contribute to neurodegeneration in tauopathies. One of the key questions to move toward therapeutic development is, which types of tau are toxic? This paper points to tau acetylated at lysine 174 as one of the toxic species.
In a comprehensive set of experiments ranging from postmortem AD brain to cultured neurons and two mouse models of tauopathy, the authors demonstrate that increases in levels of this species are associated with toxicity and decreases are beneficial. This indicates that targeting tau acetylation may have therapeutic potential.
While the authors make a strong case for this acetylation being toxic, it is likely that there is more than one toxic species of tau. Indeed, similar effects on reducing pathology and improving cognition have been seen in mice when targeting different phospho-epitopes of tau (Sigurdsson, 2014) and by targeting tau oligomers (Castillo-Carranza et al., 2014). While acetylation, phosphorylation, and oligomerization are not mutually exclusive events, it may take multiple approaches to effectively prevent tau toxicity in humans.
References:
Sigurdsson EM. Tau immunotherapy and imaging. Neurodegener Dis. 2014;13(2-3):103-6. Epub 2013 Sep 11 PubMed.
Castillo-Carranza DL, Sengupta U, Guerrero-Muñoz MJ, Lasagna-Reeves CA, Gerson JE, Singh G, Estes DM, Barrett AD, Dineley KT, Jackson GR, Kayed R. Passive immunization with Tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J Neurosci. 2014 Mar 19;34(12):4260-72. PubMed.
RIKEN Center for Brain Science
This is a finely designed, beautiful work. A simple concern: Does replacement of the lysine residue by glutamine completely mimic acetylation in structural biology terms? At least biochemically, acetylated lysine and glutamine are quite different.
KULeuven
Interesting findings. They illustrate perfectly the major problem that troubles fundamental research into protein tau, in the first instance in AD but evidently in all tauopathies.
As opposed to APP and amyloid, we do know the major physiological function of protein tau: spacing of microtubules to allow transport by motor proteins in both directions. We fail to understand the molecular changes that regulate the dynamics of binding of protein tau to microtubules, and eventually to other proteins and structures, i.e., membranes and/or F-actin.
Free protein tau is a floppy, non-structured protein, and its maximum of 441 amino acids offer a vast array of potential post-translational modifications (PTM). My classic example: If we account for only 15 physiologically relevant phosphorylation sites, the number of different "normal" tau isoforms already reaches 215, or more than 32,000.
Including all other known PTM, and for sure more are still to be discovered, increases that already vast number exponentially. It is evident that all sorts of correlations are observed—some very tight and others more loose. This paper is a fine example of such studies, but only one of many.
Nevertheless, correlation is neither cause nor consequence: the tight correlation between chocolate consumption and Nobel Prize winners, or the arrival of storks and the number of births are of no relevance for the members of these equations.
The reported acetylation is to be regarded in that light. Mice with a specific defect in the enzyme or the residue on tau will shed some more light on what eventually will turn out to be the neuro/synapto-toxic species in ailing brain, in any or all of the many tauopathies.
The excellent data presented in studies like this and others need to be confirmed and followed up tenaciously and stringently to finally answer the primary questions!
Make a Comment
To make a comment you must login or register.