New PET Ligand Captures α-Synuclein in Parkinson’s, MSA Brains
Quick Links
Crafting a PET tracer to detect α-synuclein deposits in the brain has been an uphill struggle. Now, scientists led by Hironobu Endo and Makoto Higuchi at the National Institutes for Quantum Science and Technology in Chiba, Japan, describe a new ligand, C05-05, that is a step in the right direction. In the June 5 Neuron, they report that, in brain tissue from mice, marmosets, and people, the molecule bound α-synuclein fibrils. On PET, C05-05 detected aggregates in multiple system atrophy, dementia with Lewy bodies, and Parkinson’s disease. Tracer brightness in the midbrain correlated with motor symptom severity. Still, C05-05 also bound to neurofibrillary tangles and amyloid plaques, albeit more weakly than to α-synuclein aggregates. The authors are trying to tweak the ligand to make it more selective.
- A new PET ligand, C05-05, binds α-synuclein fibrils.
- It lit up the midbrain in PD and DLB; the putamen and cerebellum in MSA.
- High midbrain C05-05 uptake correlated with worse motor symptoms.
“That C05-05 images α-synuclein in PD demonstrates the target density is high enough to see with PET, a question that has been raised for years,” Robert Mach of the University of Pennsylvania in Philadelphia, told Alzforum.
Francesca Capotosti of AC Immune compared C05-05 to other synuclein tracers for MSA, a disease with dense α-synuclein aggregates. “It's nice to see molecules coming from different chemical structures have a common pattern in what they show on PET,” she said. “This gives confidence to the field that what is imaged corresponds to pathological α-synuclein.”
PD, DLB, and MSA all start with motor symptoms, making them difficult to distinguish, especially at early stages. They can only be definitively diagnosed postmortem, and while detecting α-synuclein aggregates in cerebrospinal fluid and serum is possible, it is not quite up to snuff for diagnostic use (Apr 2023 conference news; Aug 2023 conference news). Measuring the spread of synuclein through a person’s brain using PET imaging could aid in disease staging and diagnosis.

Better Chemistry? Scientists synthesized C05-05 by changing the linker (red) and modifying the benzothiazole ring (left) in PBB3. [Courtesy of Endo et al., Neuron, 2024.]
Scientists have developed α-synuclein PET tracers, but they have limitations (Aug 2023 conference news). One, called F0502B, poorly slipped into the brains of rhesus macaques, while Merck's MK-7337 had off-target binding in monkey cerebella. AC Immune’s ACI-12589 detects α-synuclein aggregates in MSA, but not those in PD, which are much sparser. Capotosti says the company plans to test a more sensitive derivative, dubbed ACI-15916, in a first-in-human trial in PD beginning early next year.
Aiming to develop a better ligand, co-first authors Endo and Maiko Ono modified the tau PET tracer PBB3 because it also bound the β sheets of α-synuclein inclusions (Koga et al., 2017; Perez-Soriano et al., 2017). The scientists swapped a double bond for a triple bond in a hydrocarbon chain linking two aromatic rings in the tracer, then added various functional groups to the rings to create the C05 series of compounds (image above). One, called C05-05, stood out because it tightly bound Lewy bodies and neurites in amygdala tissue from DLB cases. However, it also bound neurofibrillary tangles and amyloid plaques in middle frontal gyrus tissue from a person who had had AD (image below).

Not There Yet. Fluorescence microscopy showed C05-05 bound Lewy bodies and neurites (top left) in DLB brain tissue better than did PBB3 (bottom left), but it also tagged neurofibrillary tangles (arrowheads) and amyloid plaques (arrows) in AD tissue (right). [Courtesy of Endo et al., Neuron, 2024.]
To see how the tracer behaved in vivo, Endo and Ono first used wild-type mice that had been injected with mouse α-synuclein fibrils to seed aggregates throughout the brain. Six months later, the scientists injected an 18F-C05-05 into a vein, then scanned the brains using micro-PET. The highest tracer uptake occurred in the striatum, cortex, and amygdala, areas with abundant synuclein aggregates seen via immunofluorescence (image below). The cerebellum, which had no synuclein inclusions, bound little tracer. The tracer bound off-target in the corpus callosum and the hippocampus, in both α-synuclein and wild-type mice.

Mouse PET. In PET scans (left), 18F-C05-05 was retained in the striata (top) and neocortices (middle) of α-synuclein-seeded mice (left) but not of wild-type mice (right). The cerebellum (bottom) bound no tracer. Autoradiographs and immunofluorescence (right) showed the tracers accumulated in the same areas (arrows) as phosphorylated α-synuclein. The numerals 1 and 2 indicate striatum and corpus callosum, respectively. [Courtesy of Endo et al., Neuron, 2024.]
The scientists next tested the tracer in a nonhuman primate model; that is, marmosets that had α-synuclein fibrils seeded in the brain. 18F-C05-05 accumulated in the caudate nucleus, putamen, and substantia nigra, all places with dense α-synuclein aggregates detected by immunohistochemistry.
What about people? First, the scientists used autoradiography to test tracer binding in amygdala from a DLB donor and in basal ganglia tissue from people who had had MSA or PD. 18F-C05-05 bound all slices and tracked with the level of α-synuclein aggregates, meaning no labeling in cases of mild pathology and intense binding with severe pathology.
Endo and colleagues then tested the tracer in 10 people with PD or DLB and eight controls. The amount of tracer in the brain peaked within minutes after intravenous injection, then fell by half in most areas by 50 minutes. However, in people with PD or DLB, radioactivity remained high in the midbrain, home of the substantia nigra, after two hours (image below). Higher midbrain binding correlated with worse motor impairment, as measured by the revised Unified PD Rating Scale. Capotosti called this correlation remarkable, given such small sample sizes.
18F-C05-05 also lit up the putamen and middle cerebellar peduncle, which connects the cerebellum to the pons, in three people with MSA (image below). These areas are known hotspots for glial cytoplasmic inclusions, the characteristic oligodendrocyte α-synuclein pathology found in MSA (reviewed by Castellani, 1998). The data show that the ligand reacts with α-synuclein aggregates in all three synucleinopathies. “Our work has offered a benchmark for the development of α-synuclein PET tracers by showing that a radiotracer with a dissociation constant of about 1 nM can visualize target pathologies in PD and DLB at moderate and advanced stages,” Higuchi told Alzforum.
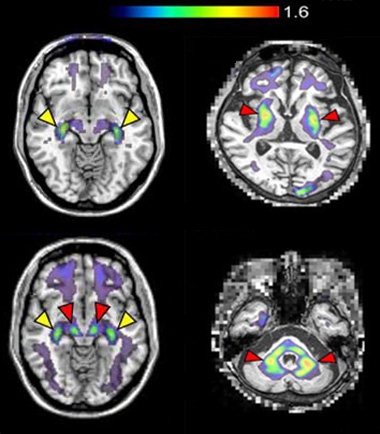
Illuminating Synuclein. While 18F-C05-05 bound the choroid plexus (yellow arrows) in healthy controls (top left), it only lit up the midbrain in people with PD or DLB (red arrows, bottom left). The tracer also bound the putamen (red arrows, top right) and middle cerebellar peduncle (red arrows, bottom right) in people with MSA. [Courtesy of Endo et al., Neuron, 2024.]
Other scientists said C05-05 needs more work before it would be suitable for the clinic. Behrooz Yousefi at Philipps University of Marburg, Germany, noted that slow brain uptake and rapid metabolism, at least in mice, make the tracer less than ideal, while Oskar Hansson, Lund University, Sweden, feels the specificity needs to be higher. “I think the current tracer is a great step forward, but the overlap between [tracer uptake in] controls and patients with PD is far too great for it to be a reliable diagnostic marker,” he wrote.
Capotosti was a bit disappointed by C05-05’s affinity for tangles and plaques, given that more than half of people with PD have these co-pathologies (Irwin et al., 2017). “The lack of selectivity will represent a major liability in further validating the tracer as a diagnostic marker,” she said.
Mach noted that 18F-C05-05 binds α-synuclein near tyrosine 39, a hotspot for phosphorylation or nitration, which he predicted might hinder tracer binding in vivo, but it seems this is not the case for this compound. “We changed our strategy for developing α-synuclein PET ligands to focus on ones that bind near tyrosine 39,” he told Alzforum.
Higuchi and colleagues are making derivatives of C05-05, aiming to find molecules more selective for α-synuclein inclusions.
As for what’s to come for the field, about half a dozen α-synuclein PET tracers in clinical trials are set to read out next year. These include F0502B, Merck’s MK-7337, and AC Immune’s ACI-12589, as well as two tracers, HY215 and M503, that Mach is developing (Kim et al., 2023). “Twenty-twenty-five will be a big year for α-synuclein imaging,” he predicted. —Chelsea Weidman Burke
References
News Citations
- Synuclein Assay Passes the Sniff Test—What of Other Seeds?
- Finally, a Diagnostic Marker for Lewy Body Disease?
- Spying on α-Synuclein Inclusions: PET Tracers Inch Closer to Success
Paper Citations
- Koga S, Ono M, Sahara N, Higuchi M, Dickson DW. Fluorescence and autoradiographic evaluation of tau PET ligand PBB3 to α-synuclein pathology. Mov Disord. 2017 Jun;32(6):884-892. Epub 2017 Apr 25 PubMed.
- Perez-Soriano A, Arena JE, Dinelle K, Miao Q, McKenzie J, Neilson N, Puschmann A, Schaffer P, Shinotoh H, Smith-Forrester J, Shahinfard E, Vafai N, Wile D, Wszolek Z, Higuchi M, Sossi V, Stoessl AJ. PBB3 imaging in Parkinsonian disorders: Evidence for binding to tau and other proteins. Mov Disord. 2017 Jul;32(7):1016-1024. Epub 2017 Jun 1 PubMed.
- Castellani R. Multiple system atrophy: clues from inclusions. Am J Pathol. 1998 Sep;153(3):671-6. PubMed.
- Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, Lee EB, Van Deerlin VM, Lopez OL, Kofler JK, Nelson PT, Jicha GA, Woltjer R, Quinn JF, Kaye J, Leverenz JB, Tsuang D, Longfellow K, Yearout D, Kukull W, Keene CD, Montine TJ, Zabetian CP, Trojanowski JQ. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol. 2017 Jan;16(1):55-65. PubMed.
- Kim HY, Chia WK, Hsieh CJ, Saturnino Guarino D, Graham TJ, Lengyel-Zhand Z, Schneider M, Tomita C, Lougee MG, Kim HJ, Pagar VV, Lee H, Hou C, Garcia BA, Petersson EJ, O'Shea J, Kotzbauer PT, Mathis CA, Lee VM, Luk KC, Mach RH. A Novel Brain PET Radiotracer for Imaging Alpha Synuclein Fibrils in Multiple System Atrophy. J Med Chem. 2023 Sep 14;66(17):12185-12202. Epub 2023 Aug 31 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Endo H, Ono M, Takado Y, Matsuoka K, Takahashi M, Tagai K, Kataoka Y, Hirata K, Takahata K, Seki C, Kokubo N, Fujinaga M, Mori W, Nagai Y, Mimura K, Kumata K, Kikuchi T, Shimozawa A, Mishra SK, Yamaguchi Y, Shimizu H, Kakita A, Takuwa H, Shinotoh H, Shimada H, Kimura Y, Ichise M, Suhara T, Minamimoto T, Sahara N, Kawamura K, Zhang MR, Hasegawa M, Higuchi M. Imaging α-synuclein pathologies in animal models and patients with Parkinson's and related diseases. Neuron. 2024 Aug 7;112(15):2540-2557.e8. Epub 2024 Jun 5 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.