Might SORL1 Bind Tau in Glia, Fuel Its Aggregation?
Quick Links
Over the last few decades, evidence has emerged to peg the sortilin-related receptor, SORL1, as a risk factor for Alzheimer’s disease and even as the fourth gene for familial AD. SORL1 helps traffic amyloid precursor protein through the endolysosomal system, where delays can increase the production of Aβ. Could the receptor be involved in tau pathology, too? Yes, say scientists led by Bradley Hyman at Massachusetts General Hospital in Charlestown and Dudley Strickland of the University of Maryland, Baltimore. In the April 22 Journal of Biological Chemistry, they reported that, in vitro, human recombinant SORL1 bound tau and that the two co-localized in neuroglioma cells. What’s more, SORL1 seems to have a hand in tau seeding because knocking the receptor down reduced tau aggregation, while expressing the N1358S variant increased it. This might explain why this mutation would increase the risk for AD. Scientists have been unsure how, or if, the variant affects SORL1 function.
- In surface plasmon resonance experiments, tau binds SORL1.
- In neuroglioma cells, the two co-localize.
- Knocking down SORL1 limits tau seeding; overexpressing SORL1 exacerbates it.
- So does the SORL1 N1358S risk variant.
Kenneth Kosik at the University of California, Santa Barbara, called this a well-executed study. “In essence, the problem we face is that, internalized via LRP1, through which it enters endosomes, tau must then escape the endosome before it can assemble into cytoplasmic neurofibrillary tangles. This new discovery offers evidence that tau is handed off to SORL1 inside the cell … and most importantly, that this interaction promotes seeding,” he wrote (comment below).
A transmembrane protein, SORL1 primarily resides on endosomes. It shuttles cargo, such as amyloid precursor protein, from the cell surface, though only 10 percent of total SORL1 associates with the cell membrane at any one time (Jul 2023 news; Jacobsen et al., 2001). Since the receptor contains LDL ligand-binding domains similar to those in LDL receptor-related protein 1 (LRP1), a proposed cell-surface tau receptor, the authors wondered if SORL1 binds tau, too (Mar 2020 news; Cooper et al., 2021)
To find out, co-first authors Joanna Cooper at U Maryland and Aurelien Lathuiliere of Mass General turned to surface plasmon resonance spectroscopy, which measures the interaction between one immobilized biomolecule on a thin surface and another by detecting changes in light bouncing off that surface. They found recombinant human SORL1 latched tightly to recombinant human tau with a dissociation constant of 59 nM.
To see if the two bound in cells, Cooper and Lathuiliere tracked radiolabeled tau in H4 neuroglioma cells, which express both LRP1 and SORL1. Adding an anti-LRP1 antibody to the medium blocked most tau uptake into the cells, whereas knocking down SORL1 had no effect. Still, 17 percent of fluorescently labeled tau co-localized with SORL1, as seen using confocal microscopy, indicating that the two bind once tau is inside the cell (image below).
Curious if this binding happens within endosomes, the scientists tested it at pH 5.5, the acidity of those organelles. Indeed, in plasmon resonance experiments, they bound as tightly at pH 5.5 as at pH 7.4. “We think that tau dissociates from LRP1 at endosomal pH (~pH 5.5) and is picked up by SORL1,” Strickland told Alzforum. Strickland said they are now mapping the co-localization using markers for various organelles.
Gregory Petsko of Brigham and Women’s Hospital in Boston had a different interpretation. Since tau is not an integral membrane protein, he thinks that SORL1 must meet tau at the cell surface where the pH is more neutral. “It is that SORL1 binds tau at pH 7.4 that is the important finding,” he wrote (comment below).
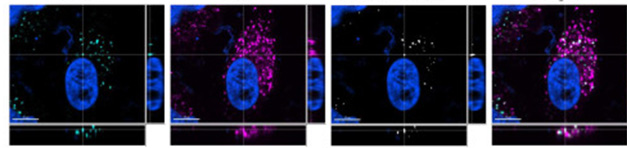
SORL1 Meets Tau. In a neuroglioma cell, SORL1 (turquoise) co-localized (white) with tau (purple). [Courtesy of Cooper et al., Journal of Biological Chemistry, 2024.]
For his part, Marc Diamond, UT Southwestern, Dallas, also wondered if the binding takes place in the endosome, questioning the specificity of the co-localization data. “If you co-stain for LDL receptors, or a dozen related receptors, how often would they co-localize with tau that has been taken up?” he asked. “I would want to see other transmembrane receptors that could potentially bind tau as negative controls,” he added (comment below).
How would binding to SORL1 fit with tau biology? To see if the liaison affected tau aggregation, Cooper and Lathuiliere studied seeding in reporter cell lines expressing fluorescent P301S tau (Oct 2014 news). In cells overexpressing SORL1, tau extracted from AD brain tissue seeded 13 times more tau aggregates than in control cells. In contrast, silencing SORL1 in neuroglioma cells expressing the same fluorescent P301S tau biosensor halved tau aggregation. The authors concluded that SORL1 encourages tau seeding.
Next, the scientists turned their attention to the SORL1 mutant N1358S, which was found in a French family with a history of autosomal dominant AD but with no mutations in APP or presenilins 1 or 2.
HEK cells overexpressing N1358S SORL1 did not take in any more tau than cells expressing the wild-type receptor. However, they seeded 1.5-fold more tau fibrils. The point mutation lies in the LDL ligand binding region, one that is also found in LRP1, suggesting that the domain is a binding site for tau. The authors believe that N1358S might weaken that binding—though they did not test it directly with surface plasmon resonance—possibly allowing the protein to escape the endosome and aggregate (image below). To the authors, this suggests that N1358S might be pathogenic.
Petsko was not convinced. “The clinical pathogenicity of this variant is not well-established,” he wrote, noting that the asparagine to serine switches are generally benign. Indeed, only one of three prediction algorithms pegged N1358S as pathogenic (Apr 2012 news; Nov 2022 news).
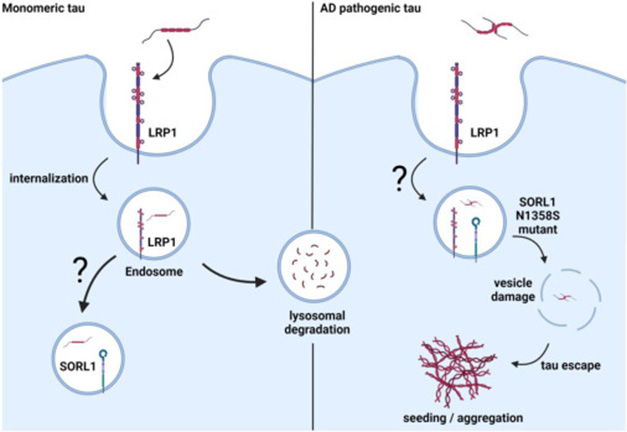
Sordid Model. Tau (red, left), taken into cells via LRP1, is shunted to SORL1 in endosomes or degraded by lysosomes. In AD, pathogenic forms of tau (right) also enter cells through LRP1 but escape degradation and slip from SORL1’s grip into the cytosol, where they aggregate. The N1358S variant exacerbates this process. [Courtesy of Cooper et al., Journal of Biological Chemistry, 2024.]
All told, dysfunctional SORL1 might be a double-edged sword, releasing tau into the cytoplasm where it might aggregate, while retaining APP in endosomes where it can be processed to release Aβ, as reported previously. “This increases the attractiveness of SORL1 as a potential drug target,” wrote Sam Gandy of the Icahn School of Medicine, New York (comment below). “It will be interesting to see whether the pharmacological chaperones that stabilize SORL1 and modulate amyloidogenesis have a beneficial, neutral, or detrimental effect on SORL1 promotion of tau seeding,” he added.
One such chaperone, called R55, purportedly binds to and stabilizes the retromer complex, which maintains SORL1 trafficking of APP (Apr 2014 news). Petsko and Scott Small of Columbia University in New York, who co-developed R55, told Alzforum that it has not been tested in the clinic because it does not bind tightly enough to the retromer and because it is unstable in vivo. “I think that it would be interesting to test to see whether R55 modulates tau seeding even if it is not the world’s best lead compound,” said Gandy.—Chelsea Weidman Burke
References
Mutation Interactive Images Citations
News Citations
- When Missense Variants Derail SORL1 Traffic, Destination Is Dementia
- Tau Receptor Identified on Cell Surface
- Cellular Biosensor Detects Tau Seeds Long Before They Sprout Pathology
- New Genetic Insights Into AD: SORL1 and Natural Selection
- Rare Variants in Lipid Transporter Genes Increase Risk for Alzheimer’s Disease
- Could Bolstering the Retromer Thwart Alzheimer’s?
Mutations Citations
Paper Citations
- Jacobsen L, Madsen P, Jacobsen C, Nielsen MS, Gliemann J, Petersen CM. Activation and functional characterization of the mosaic receptor SorLA/LR11. J Biol Chem. 2001 Jun 22;276(25):22788-96. Epub 2001 Apr 9 PubMed.
- Cooper JM, Lathuiliere A, Migliorini M, Arai AL, Wani MM, Dujardin S, Muratoglu SC, Hyman BT, Strickland DK. Regulation of tau internalization, degradation, and seeding by LRP1 reveals multiple pathways for tau catabolism. J Biol Chem. 2021 Jan-Jun;296:100715. Epub 2021 Apr 28 PubMed.
Further Reading
Primary Papers
- Cooper JM, Lathuiliere A, Su EJ, Song Y, Torrente D, Jo Y, Weinrich N, Sales JD, Migliorini M, Sisson TH, Lawrence DA, Hyman BT, Strickland DK. SORL1 is a receptor for tau that promotes tau seeding. J Biol Chem. 2024 Jun;300(6):107313. Epub 2024 Apr 23 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.