Microglial Transplants Reverse Age-Related Pathology in Mice
Quick Links
In diseases driven by dysfunctional microglia, could replacing them with healthy versions prevent, or even reverse, pathology? Yes, suggest two papers in the June 18 Neuron. Both describe how mice completely devoid of microglia develop astrogliosis, reactive oligodendrocytes, neurodegeneration, white-matter atrophy, and calcification of the thalamus from middle age. These symptoms mirror a rare neurodegenerative disease in people, which goes by the mouthful adult-onset leukoencephalopathy with axonal spheroids and pigmented glia. ALSP affects people who have mutations in the gene for colony stimulating factor 1 receptor, which is essential for microglial proliferation. By reintroducing microglia to mice devoid of these cells, the scientists were able to prevent, even reverse, ALSP-like pathologies.
- Without microglia, mice get astrogliosis and neurodegeneration.
- Their pathology resembles that of leukoencephalopathy in people.
- Replenishing microglia in young mice prevents this.
- In adult mice, it reverses it.
In one of the studies, scientists led by first author David Munro and Josef Priller at the University of Edinburgh transplanted mouse microglia. In the other, Hayk Davtyan, Mathew Blurton-Jones, and colleagues at the University of California, Irvine, gave human microglia to a humanized version of the microglia-free mice. Both strategies worked.
“Overall, this is very exciting and certainly something we should continue pursuing in the field,” wrote Anna Martinez-Muriana and Renzo Mancuso, VIB-Center for Molecular Neurology, Antwerp, Belgium. The prospects for basic research impressed Nóra Baligács and Bart de Strooper of KU Leuven, Belgium. “This presents an exciting opportunity to … investigate the role of microglia and their diverse signaling pathways in brain function in unprecedented ways,” they wrote (comments below). Others saw potential for replenishing failing microglia in Alzheimer’s and other neurodegenerative diseases, even though the focus in these two papers was on primary microgliopathies.
The latter arise when too few healthy microglia patrol the brain. In the case of ALSP, people develop memory problems, personality changes, and sensory deficits in their 30s and 40s. They get tremors and walk more slowly. These symptoms are rooted in reactive astrocytes, white-matter atrophy, brain calcification, blood-brain barrier dysfunction, and axonal spheroids, i.e., blebs on axons triggered by inflammation or degeneration (Konno et al., 2017; Robinson et al., 2015; reviewed by Mickeviciute et al., 2021).
Modeling ALSP in mice was difficult until scientists knocked out the fms-intronic regulatory element in the promoter that drives CSF1R gene expression. At first, the “FIRE” mice, which make no microglia, appeared surprisingly normal, even up to adulthood at 9 months (Rojo et al., 2019). Upon closer inspection, however, scientists found that 6-month-old mice roused a subset of inflammatory oligodendrocytes expressing the serine protease inhibitor Serpina3n and had lost myelin (Oct 2023 conference news). Serpina3n marks disease-associated oligodendrocytes in mouse models of neurodegenerative diseases (Kenigsbuch et al., 2022).
Munro and colleagues reported that as FIRE mice aged, they began to show more signs of ALSP. By 11 months, calcium had deposited in the thalamus, while MRI showed voids appeared in white matter of the thalamus, basal ganglia, and pons. These all worsened with age. At 12 months, some FIRE mice began dragging their hindlimbs and hunching their backs. By 18 months, half of them died. At this point, the thalamus had lost one-quarter of its neurons, with axonal spheroids dotting the hippocampus. Reactive astrocytes patrolled white-matter tracts, hippocampi, and cortices, and Serpina3n-positive oligodendrocytes multiplied throughout the white matter and thalamus (image below).
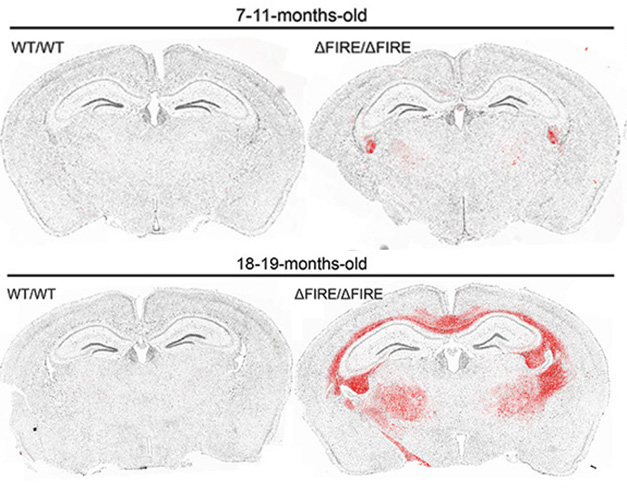
Reactive Oligos. Serpina3n-positive oligodendrocytes (red) start to gather in the white-matter tracts and thalami in adult FIRE mice (top right) and amass there by old age (bottom right). [Courtesy of Munro et al., Neuron, 2024.]
In Blurton-Jones’ lab, first author Jean Paul Chadarevian saw much the same in FIRE mice he created to support human microglial grafts. Called hFIRE, these also have no microglia. They express the human version of the CSF1R ligand, CSF1, and they lack B, T, and natural killer cells that might mount an immune response to the human microglia the scientists planned to graft.
Two-month-old hFIRE brains looked normal. By 8.5 months, Serpina3n-positive oligodendrocytes predominated in the white matter of the hippocampus, which was packed with lipids. As with the FIRE mice, astrocytosis, calcium deposits, axonal spheroids, and thalamic neurodegeneration hampered the hFIRE mice. Though Serpin3a oligodendrocytes were not detected in the thalamus, microbleeds and deposits of plasma albumin there indicated blood-brain barrier damage.
“It is remarkable that both studies find many overlapping pathological hallmarks of ALSP,” wrote Sabina Tahirovic at DZNE in Munich (comment below). “ALSP provides an ideal condition … to consider a microglia transplantation strategy.”
Transplants to the Rescue?
Munro and colleagues transplanted microglia from wild-type mice into 1-day-old FIRE pups. Nine months later, the cells had flourished throughout the brain. These brains had no calcium deposits, axonal spheroids, Serpina3n-positive oligodendrocytes, and few reactive astrocytes. They boasted 20 percent more neurons in the thalamus than untreated FIRE mice, nearly as many as wild-type.
For their part, Chadarevian and colleagues injected healthy microglia derived from human iPSCs into the hippocampi of 2-month-old hFIRE mice, before pathology. Microglia filled the brain within a month (image below). After 6.5 months, most were homeostatic, as measured by bulk RNA-Seq, and appeared to be highly ramified, as is typical for microglia in a healthy brain.

Microglia on the March. Human microglia (green) spread from the hippocampal injection site (left) throughout the brain (right) within 30 days. [Courtesy of Chadarevian et al., Neuron, 2024.]
Mirroring Munro's findings, transplanted hFIRE mice developed hardly any ALSP pathology. They had no calcium deposits, microbleeds, or axonal spheroids and as many neurons as wild-type mice. Astrogliosis and Serpina3n expression were no different from wild-types.
“Both publications demonstrate the remarkable ability of grafted microglial progenitors to colonize the entire mouse brain, adopt homeostatic signatures and functionally integrate,” wrote Gesine Saher at the Max Planck Institute for Multidisciplinary Sciences, Gottingen, Germany (comment below).
Could introducing microglia reverse established pathology in FIRE mice? Thinking about the possible therapeutic applications, Chadarevian and colleagues created iPSCs from a woman who carried the L786S CSF1R variant that causes ALSP, corrected the mutation to the L786L functional variant to create an isogenic control line, and then differentiated both lines into microglia. Six weeks after injecting the control cells, but not the L786S versions, into the hippocampi of 4-month-old hFIRE mice, axonal spheroids had disappeared, calcium crystals had dissolved, astrocytes were calmed, and oligodendrocyte Serpina3n expression fell by a third (image below). “I was pleasantly surprised how quickly pathology reversed,” Blurton-Jones told Alzforum.
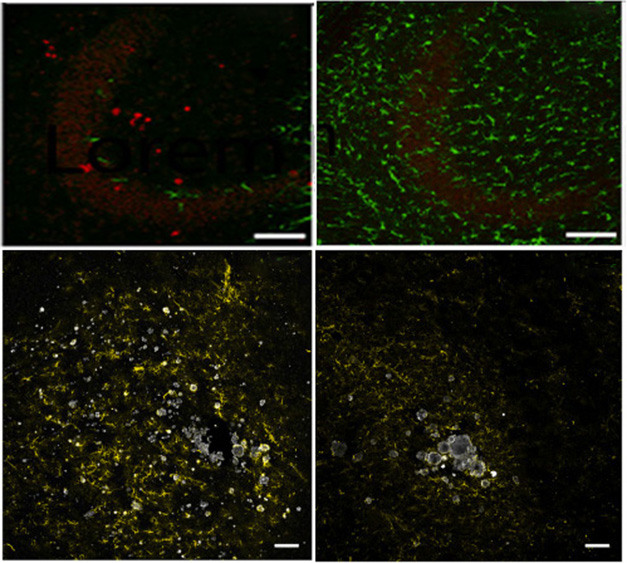
Reverse It. In hFIRE mice, injected human microglia (green) carrying the L786S CSF1R variant are sparse after 6 weeks (left, top). Axonal spheroids (red), astrogliosis (yellow, bottom), and calcium deposits (white, bottom) remain. In contrast, L786L CSF1R microglia flourish (top right), axonal spheroids disappear, astrocytes calm down, and calcium deposits shrink (bottom right). [Courtesy of Chadarevian et al., Neuron, 2024.]
Blurton-Jones thinks this could be a valuable therapeutic strategy. However, these experiments don’t exactly mimic what happens in people because they already have microglia in their brains, even if they are not functioning normally.
To better model how injected microglia might take over from an existing population, Blurton-Jones and colleagues waited six months until the weak CSF1R mutant human microglia populated the hFIRE brain, then added CRISPR-corrected microglia. They are now testing if the latter fill the brain and rescue pathology.
Baligács and de Strooper think this could become a powerful therapeutic approach, particularly for ALSP, where microglia numbers and proliferation are low. “In other conditions, where microglial numbers are not affected, treatments to deplete endogenous microglia before transplantation may be necessary,” they wrote.
Could this work in Alzheimer’s disease, where certain microglial genotypes may be protective? “These suggestions remain futuristic for now. We still lack a clear understanding of the precise role of microglia in Alzheimer’s, and the practical challenges of implementing cell therapies in humans will require significant and persistent research efforts,” they wrote (comment below).
California-based NovoGlia, co-founded by Blurton-Jones, has grants from California’s Stem Cell Agency to work on microglia transplants for ALSP.—Chelsea Weidman Burke
References
News Citations
Paper Citations
- Konno T, Yoshida K, Mizuno T, Kawarai T, Tada M, Nozaki H, Ikeda SI, Nishizawa M, Onodera O, Wszolek ZK, Ikeuchi T. Clinical and genetic characterization of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia associated with CSF1R mutation. Eur J Neurol. 2017 Jan;24(1):37-45. Epub 2016 Sep 29 PubMed.
- Robinson JL, Suh E, Wood EM, Lee EB, Coslett HB, Raible K, Lee VM, Trojanowski JQ, Van Deerlin VM. Common neuropathological features underlie distinct clinical presentations in three siblings with hereditary diffuse leukoencephalopathy with spheroids caused by CSF1R p.Arg782His. Acta Neuropathol Commun. 2015 Jul 4;3:42. PubMed.
- Mickeviciute GC, Valiuskyte M, Plattén M, Wszolek ZK, Andersen O, Danylaité Karrenbauer V, Ineichen BV, Granberg T. Neuroimaging phenotypes of CSF1R-related leukoencephalopathy: Systematic review, meta-analysis, and imaging recommendations. J Intern Med. 2022 Mar;291(3):269-282. Epub 2021 Dec 22 PubMed.
- Rojo R, Raper A, Ozdemir DD, Lefevre L, Grabert K, Wollscheid-Lengeling E, Bradford B, Caruso M, Gazova I, Sánchez A, Lisowski ZM, Alves J, Molina-Gonzalez I, Davtyan H, Lodge RJ, Glover JD, Wallace R, Munro DA, David E, Amit I, Miron VE, Priller J, Jenkins SJ, Hardingham GE, Blurton-Jones M, Mabbott NA, Summers KM, Hohenstein P, Hume DA, Pridans C. Deletion of a Csf1r enhancer selectively impacts CSF1R expression and development of tissue macrophage populations. Nat Commun. 2019 Jul 19;10(1):3215. PubMed.
- Kenigsbuch M, Bost P, Halevi S, Chang Y, Chen S, Ma Q, Hajbi R, Schwikowski B, Bodenmiller B, Fu H, Schwartz M, Amit I. A shared disease-associated oligodendrocyte signature among multiple CNS pathologies. Nat Neurosci. 2022 Jul;25(7):876-886. Epub 2022 Jun 27 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Munro DA, Bestard-Cuche N, McQuaid C, Chagnot A, Shabestari SK, Chadarevian JP, Maheshwari U, Szymkowiak S, Morris K, Mohammad M, Corsinotti A, Bradford B, Mabbott N, Lennen RJ, Jansen MA, Pridans C, McColl BW, Keller A, Blurton-Jones M, Montagne A, Williams A, Priller J. Microglia protect against age-associated brain pathologies. Neuron. 2024 Aug 21;112(16):2732-2748.e8. Epub 2024 Jun 18 PubMed.
- Chadarevian JP, Hasselmann J, Lahian A, Capocchi JK, Escobar A, Lim TE, Le L, Tu C, Nguyen J, Kiani Shabestari S, Carlen-Jones W, Gandhi S, Bu G, Hume DA, Pridans C, Wszolek ZK, Spitale RC, Davtyan H, Blurton-Jones M. Therapeutic potential of human microglia transplantation in a chimeric model of CSF1R-related leukoencephalopathy. Neuron. 2024 Aug 21;112(16):2686-2707.e8. Epub 2024 Jun 18 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.