Longitudinal Tau PET Links Aβ to Subsequent Rise in Cortical Tau
Quick Links
The growth of tau tangle pathology in the brain’s cortex only happens in people who also have Aβ plaques, and it correlates with cognitive decline. These conclusions are supported by the largest, multicenter, longitudinal tau PET study conducted to date, published in Brain on April 22. Researchers led by Michael Pontecorvo at Avid Radiopharmaceuticals in Philadelphia reported that cortical tau tracer uptake revved up over 18 months in people who had had positive amyloid-PET scans at baseline. What’s more, the more tau pathology a person had at the start of the study, the faster it increased and the more steeply their cognition declined over the next 18 months.
- Cortical tangle burden increased only in people with amyloid.
- The more tau there was at the start, the more it grew over 18 months.
- Both baseline tau and rise of tau correlated with cognitive decline.
“The data provide additional support for a strong, longitudinal relationship between tau and changes in cognition, which further establishes tau PET as an important outcome measure in clinical trials,” commented Gil Rabinovici of the University of California, San Francisco.
The study lends support to the idea that Aβ deposition is a prerequisite for tau’s expansion into the cortex, a move that coincides with the onset of clinical symptoms. Older, cross-sectional studies have drawn similar conclusions (Mar 2016 news; Aug 2016 news).
More recently, smaller tau PET longitudinal studies reported that tau’s cortical expansion is mostly limited to people who have amyloid plaques, that its regional distribution correlates with the severity of cognitive impairment, and that baseline tau levels predict tau’s subsequent rise (Jack et al., 2018; Cho et al., 2019; Sintini et al., 2019). Another recent study saw tau increase in some regions even among older people without Aβ deposition, although it rose much faster in people with Aβ (Harrison et al., 2019).
Longitudinal studies are crucial to teasing out the relationships between Aβ, tau, and cognition. Pontecorvo and colleagues at Avid, the makers of flortaucipir (aka 18F-AV1451) set out to investigate these questions in people at different points along the AD spectrum. They enrolled 202 participants at 25 sites in the U.S. who received baseline amyloid-PET and tau PET scans. Of these, 57 were cognitively normal, 97 had mild cognitive impairment, and 48 had possible or probable AD. One hundred and sixty-eight of the participants had a second tau PET scan at nine months, while 145 had a third at 18 months. Participants took cognitive tests at each visit as well. The researchers previously published baseline results from their cohort; the current study includes follow-up data from nine and 18 months (Pontecorvo et al., 2017).

Aβ Unleashes Tau. For the most part, tau tracer uptake increased only in people with positive amyloid-PET scans (red) at baseline. [Courtesy of Pontecorvo et al., Brain, 2019.]
Of the 145 people who had clinical MCI or AD at baseline, 79 were amyloid-PET-positive, and 66 were negative. Of the 57 cognitively normal people, only five were Aβ-positive. People who had evidence of amyloid deposition also had higher baseline tau tracer uptake. In the Aβ-positive group, the global cortical flortaucipir standard uptake value ratio (SUVR) increased from baseline to nine months and 18 months. However, with a few exceptions, tau tracer uptake did not budge over that time period in the Aβ-negative group, regardless of cognitive status. Within the Aβ-positive group, the researchers found that baseline tau tracer uptake correlated with change in tau burden over 18 months. Younger amyloid-positive people, on average, had higher baseline tau than older amyloid-positive people.
Teasing apart the relationship between baseline tau and its subsequent rise, the researchers found that people whose baseline tau was in the lowest quartile of the group had little subsequent change in tau burden over 18 months, while those whose tau was in the second quartile at baseline had the largest increases in the inferior and lateral temporal cortex and posterior cingulate. For those in the third quartile, tau increased predominantly in the posterior lateral temporal, lateral occipital, and medial and lateral parietal cortex. Finally, in those with the highest baseline tau tracer uptake, tau increased further in the medial parietal and frontal cortex. The expansion of tau pathology from temporal to parietal to frontal regions aligns well with Braak staging gleaned from postmortem studies, the researchers concluded.
However, Val Lowe of the Mayo Clinic in Rochester, Minnesota, said that tau tracer uptake patterns do not match perfectly within the Braak staging. He noticed that even in people within the lower two quartiles of baseline tau, tracer uptake increased in frontal regions. “Very early in the disease, we notice tau tracer uptake in regions not predicted by postmortem studies to be affected,” he said. Lowe believes it is important to analyze tau PET data without preconceived notions of how tau might be spreading.
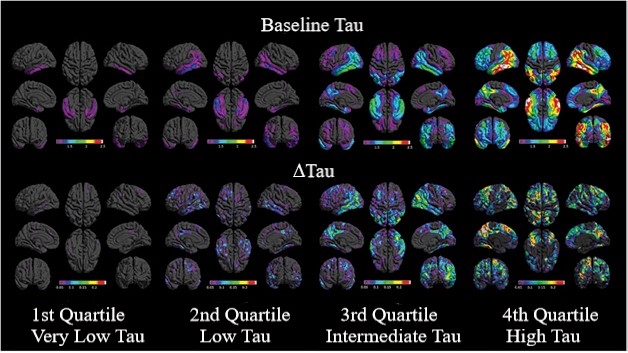
More to Start, More Spread. Distribution of baseline tau (top) and change in tau (bottom). Regional patterns of uptake and change are averaged over participants in each quartile of baseline global tau burden, each viewed from eight brain angles. [Courtesy of Pontecorvo et al., Brain, 2019.]
Both baseline tau SUVR and the magnitude of tau change over 18 months correlated with slippage on the mini mental state examination (MMSE). The relationship was not overly strong—change in tau accounted for 30 percent of the variance in cognitive scores. However, the authors think this modest relationship could result from a threshold effect, as all but one Aβ-positive person with at least some increase in tau over 18 months declined on the tests. Baseline tau burden also predicted slippage on the Alzheimer’s Disease Assessment Scale 11-item cognitive subscale (ADAS-Cog11), and the Pfeffer Functional Activities Questionnaire (FAQ).
The strong relationship between baseline tau and its subsequent expansion in the cortex makes baseline tau a candidate metric for clinical trial selection, said Stephen Salloway of Butler Hospital in Providence, Rhode Island, a study co-author and site investigator. Researchers might use cortical tau SUVR to select participants whose tau levels are on the cusp of change, upping their chances of detecting a response to drugs targeting tau. Because baseline tau also correlates with cognitive decline, it could be useful in selecting participants most likely to have a cognitive benefit from treatment, he added.
Lowe agreed that this study strongly supports using tau PET for trial selection and tracking response to treatment. Compared to cerebrospinal fluid measures of tau, which have large overlaps between clinical groups, tau PET offers a more informative marker of disease stage and impending cognitive decline, Lowe said.—Jessica Shugart
References
News Citations
- Tau PET Aligns Spread of Pathology with Alzheimer’s Staging
- Brain Imaging Suggests Aβ Unleashes the Deadly Side of Tau
Paper Citations
- Jack CR Jr, Wiste HJ, Schwarz CG, Lowe VJ, Senjem ML, Vemuri P, Weigand SD, Therneau TM, Knopman DS, Gunter JL, Jones DT, Graff-Radford J, Kantarci K, Roberts RO, Mielke MM, Machulda MM, Petersen RC. Longitudinal tau PET in ageing and Alzheimer's disease. Brain. 2018 May 1;141(5):1517-1528. PubMed.
- Cho H, Choi JY, Lee HS, Lee JH, Ryu YH, Lee MS, Jack CR Jr, Lyoo CH. Progressive tau accumulation in Alzheimer's disease: two-year follow-up study. J Nucl Med. 2019 Mar 29; PubMed.
- Sintini I, Martin PR, Graff-Radford J, Senjem ML, Schwarz CG, Machulda MM, Spychalla AJ, Drubach DA, Knopman DS, Petersen RC, Lowe VJ, Jack CR Jr, Josephs KA, Whitwell JL. Longitudinal tau-PET uptake and atrophy in atypical Alzheimer's disease. Neuroimage Clin. 2019;23:101823. Epub 2019 Apr 10 PubMed.
- Harrison TM, La Joie R, Maass A, Baker SL, Swinnerton K, Fenton L, Mellinger TJ, Edwards L, Pham J, Miller BL, Rabinovici GD, Jagust WJ. Longitudinal tau accumulation and atrophy in aging and alzheimer disease. Ann Neurol. 2019 Feb;85(2):229-240. Epub 2019 Jan 17 PubMed.
- Pontecorvo MJ, Devous MD Sr, Navitsky M, Lu M, Salloway S, Schaerf FW, Jennings D, Arora AK, McGeehan A, Lim NC, Xiong H, Joshi AD, Siderowf A, Mintun MA, 18F-AV-1451-A05 investigators. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain. 2017 Mar 1;140(3):748-763. PubMed.
Further Reading
Primary Papers
- Pontecorvo MJ, Devous MD, Kennedy I, Navitsky M, Lu M, Galante N, Salloway S, Doraiswamy PM, Southekal S, Arora AK, McGeehan A, Lim NC, Xiong H, Truocchio SP, Joshi AD, Shcherbinin S, Teske B, Fleisher AS, Mintun MA. A multicentre longitudinal study of flortaucipir (18F) in normal ageing, mild cognitive impairment and Alzheimer's disease dementia. Brain. 2019 Jun 1;142(6):1723-1735. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
UCSF
This study contributes important data on longitudinal tau PET changes across a large sample of individuals spanning the AD continuum from preclinical to dementia. The data provide additional support for a strong, longitudinal relationship between tau and changes in cognition, which further establishes tau PET as an important outcome measure in clinical trials. Interestingly, baseline tau measures were found to be highly correlated with both the degree and distribution of longitudinal tau PET changes, which will be important to consider in the design of future studies and drug trials.
The paper also contributes to the emerging story regarding patients with sporadic early onset AD, who have been shown by numerous groups to have much higher baseline tau and perhaps more rapid tau accumulation than patients with late-onset disease (Ossenkoppele et al., 2016; Schöll et al., 2017; Whitwell et al., 2019).
The recently launched, multisite Longitudinal Early-Onset AD Study (LEADS) will provide further insights into this important subpopulation of AD patients, who in my opinion have something very fundamental to teach us about disease pathophysiology.
References:
Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, O'Neil JP, Janabi M, Lazaris A, Cantwell A, Vogel J, Santos M, Miller ZA, Bettcher BM, Vossel KA, Kramer JH, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016 May;139(Pt 5):1551-67. Epub 2016 Mar 8 PubMed.
Schöll M, Ossenkoppele R, Strandberg O, Palmqvist S, Swedish BioFINDER study, Jögi J, Ohlsson T, Smith R, Hansson O. Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer's disease. Brain. 2017 Sep 1;140(9):2286-2294. PubMed.
Whitwell JL, Martin P, Graff-Radford J, Machulda MM, Senjem ML, Schwarz CG, Weigand SD, Spychalla AJ, Drubach DA, Jack CR Jr, Lowe VJ, Josephs KA. The role of age on tau PET uptake and gray matter atrophy in atypical Alzheimer's disease. Alzheimers Dement. 2019 May;15(5):675-685. Epub 2019 Mar 8 PubMed.
Make a Comment
To make a comment you must login or register.