Lindquist Leaves Behind Parkinson’s Interactome as Her Parting Gift
Quick Links
For the many genes and proteins involved in Parkinson’s disease, it’s a small world. Basically, they are all at least friends of friends. Leveraging connections between yeast genes to infer links between human genes, researchers led by the late and beloved Susan Lindquist of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, have just made that clear. Capping Lindquist’s research focus of the past five years of her life, her associates in academia and biotech have generated an extensive interaction map of genetic players in various synucleinopathies using TransposeNet, a computational algorithm they devised to “humanize” the yeast interactome. This bird’s-eye view, published January 25 in Cell Systems, revealed links between multiple parkinsonian risk factors and biological processes already projected to drive the disease. In particular, the map highlighted the importance of vesicular trafficking as well as mRNA processing and translation in α-synuclein toxicity. In a companion study from Lindquist’s lab, the researchers added a spatial dimension to these genetic connections by uncovering more than 200 proteins that brush shoulders with α-synuclein in the cell. They hope that TransposeNet and the physical interaction methods they developed can be used to identify other disease-relevant interactomes.

Susan Lindquist passed away October 27, 2016. [Courtesy of Ceal Capistrano /Whitehead Institute.]
“This duet of papers represents the swan song of one of the deepest thinkers into the fundamental biology of neurodegeneration, our recently departed friend Sue Lindquist, who sadly died a few months ago,” commented Gregory Petsko of Weill Cornell Medical College and Scott Small of Columbia University in New York, in a joint comment to Alzforum. “The papers illustrate the magnificent creativeness and scope of her life’s work, integrating yeast, genomic, and computational biology with symphonic elegance.” Lindquist succumbed to cancer at age 67 (see New York Times obituary).
“This body of work meant a good deal to Susan, because it provided a glimpse of how basic questions she had worked on for decades might play out one day to make a difference in the clinic,” former Whitehead researchers and co-authors on the current papers Vikram Khurana, Chee Yeun Chung, and Daniel Tardiff wrote in an accompanying tribute to their late mentor. “A basic biologist to the core, Susan was also a deeply engaged and empathic human being. It is thus not surprising that driving basic biological insights for the betterment of patients and humanity became an all-consuming goal for her in the latter part of her career.”

From Yeast to Human.
Yeast screens pinpoint genes involved in α-synuclein toxicity; after that, intermeshed yeast and human interaction maps display how disease processes connect. [Courtesy of Khurana et al., Cell Systems, 2017.]
The comprehensive interaction maps may provide a biological framework in which to place rare genetic variants discovered in patients, said Khurana, the first and co-corresponding author, who now heads a lab at Brigham and Women’s Hospital and the Harvard Stem Cell Institute, both in Boston. A greater understanding of how different genetic lesions relate to mechanisms that drive the disease may then pave the way for personalized therapies, he added.
Parkinson’s disease (PD) is the most common synucleinopathy; however, aggregates of α-synuclein crop up in a slew of disorders featuring parkinsonian symptoms, including dementia with Lewy bodies and multiple system atrophy, and indeed many cases of Alzheimer’s. While mutations or multiplications of the α-synuclein gene by itself cause α-synuclein pathology and PD, many other genetic culprits—both common and rare—have been identified that either cause or raise the risk of PD-like disorders, some unrelated to α-synuclein pathology (see Jul 2014 news). Understanding the connections between these diverse genetic factors and how they relate to α-synuclein aggregation will be key to finding effective treatments, Khurana said.
Genome-wide screens are a powerful way to find factors that enhance or suppress α-synuclein toxicity, and the humble yeast, with its supreme genetic malleability, offers a ready system in which to conduct these screens. Lindquist spearheaded this approach long ago, implicating protein trafficking as a driver of toxicity (see Dec 2003 news). Since then, Lindquist’s and other labs have used the screens to pin genetic modifiers of Aβ, TDP-43, and α-synuclein toxicity (see Oct 2011 news on Treusch et al., 2011; Apr 2008 news; Yeger-Lotem et al., 2009). Next, the researchers used the yeast genetic windfall to explore the roles of these genes in human disease and uncover other genes that may be involved (see Oct 2013 news; Dec 2013 news; Mar 2014 conference news; Aug 2010 news). Alas, relying on yeast to understand neurodegeneration has its limitations. Besides the obvious—yeast have no brains—there is the fact that there are no yeast homologs for human PD genes such as α-synuclein and LRRK2.
Bridging the Gap
In the current study, co-first authors Khurana, Jian Peng, now at the University of Illinois in Urbana-Champaign, and Chung, now at Yumanity Therapeutics in Cambridge, sought to flesh out the information they gathered from yeast screens and unearth the genes’ deeper context in humans. First, they expanded the screening in yeast. Previously, the researchers had overexpressed each gene of the yeast genome in yeast cells expressing α-synuclein, and uncovered 77 modifiers of α-synuclein toxicity. This time, they added a deletion screen, to identify genes that normally suppress α-synuclein toxicity, and a “pooled” overexpression screen, to test various genes simultaneously. Pooling makes screening more efficient so subtle modifiers can be detected, too. Together, these screens yielded 332 genes that either suppressed or enhanced α-synuclein toxicity in yeast.
To “humanize” this list, the researchers teamed up with computational biologists Peng and co-corresponding authors Bonnie Berger and Ernest Fraenkel of Massachusetts Institute of Technology to develop TransposeNet. To find human homologs of a given yeast gene, the algorithm relied on sequence and structural similarities, and also on similarities in known interaction partners. Applying TransposeNet to the whole yeast proteome assigned 4,923 yeast proteins to human homologs, and conversely predicted yeast homologs for 15,200 human proteins. The researchers used this rubric to convert their 332 yeast proteins into human homologs, then generated an interaction network between those. Finally, because far fewer connections between human proteins are known compared to the wealth of available yeast interaction data, the researchers used the yeast proteome to fill in missing connections in the sparse human one. “It’s like giving the human proteome a yeast roadmap,” Khurana told Alzforum. The network approach allowed the researchers to visualize connections not just among their original screen hits, but between human proteins that might have no yeast homolog.
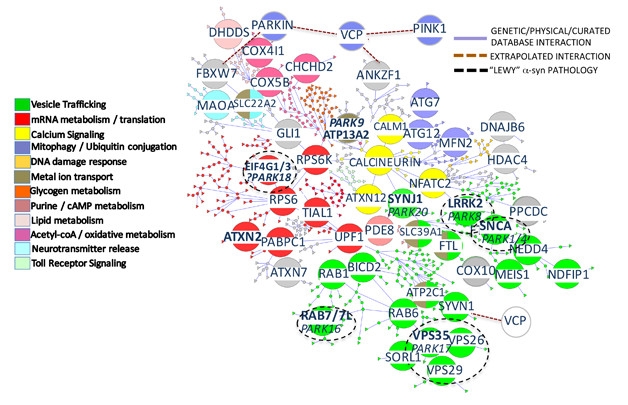
Functional Cliques. These color-coded biological processes influence the toxicity of α-synuclein. [Courtesy of Khurana et al., Cell Systems 2017.]
Lo and behold, the resulting genome-scale map of α-synuclein toxicity modifiers included several genes previously implicated in PD and other neurodegenerative diseases. Vesicular trafficking stood out as the most prominent biological process in the network, and several genes associated with typical PD, such as α-synuclein, LRRK2, and VPS35, were connected within these vesicle trafficking hubs. Proteins involved in ER-to-Golgi trafficking and ER quality control connected the two best-known PD genes, LRRK2 and α-synuclein. Importantly, neither of these genes popped up in the network without incorporating the yeast interactome to bolster the human one.
Other key members of this endosomal trafficking clique included the yeast homologs of these human genes:
- RAB7L1, an endosomal trafficking gene and leading candidate for the PARK16 locus;
- VPS35, aka PARK17, a member of the retromer complex and a causal PD gene;
- SYNJ1, aka PARK20, which also plays a role in Golgi-to-endosome trafficking;
- SORL1, an AD risk factor.
Deleting any of these genes in yeast that express normal, otherwise non-toxic levels of α-synuclein triggered growth defects. VPS35 harboring the PD-causing mutation D620N was unable to rescue deletion of the wild-type gene. Together, these findings provided support for the biological significance of endosomal trafficking, specifically retrograde transport, in PD and related disorders.
Calcium signaling genes, including calcineurin and NFAT, made an appearance smack dab in the heart of the interaction map, forming connections with several disparate biological processes. For example, calcineurin interacted with vesicular transport proteins as well as ATP13A2, aka PARK9, which encodes a type 5 ATPase implicated in metal ion homeostasis. Mutations in ATP13A2 cause a rare juvenile form of parkinsonism called Kufor-Rakeb syndrome, and the researchers found that deleting this gene in yeast boosted α-synuclein toxicity while overexpression suppressed toxicity. Interestingly, only three of 77 genes that modified toxicity in α-synuclein overexpressing cells altered toxicity in ATP13A2 deletion strains, as well. All three genes play roles in iron and manganese homeostasis, which are disrupted in Kufor-Rakeb syndrome. This supports the idea that metal ion transport represents a distinct biological pathway to α-synuclein toxicity, and perhaps to different forms of PD. Yet another set of 69 genes modified toxicity in VPS35 deletion strains as well as in α-synuclein overexpressors, indicating that vesicular trafficking plays a different, and perhaps more important role in typical forms of PD.
That mRNA translation and processing emerged as central to α-synuclein toxicity was the most intriguing outcome from the screens, according to Khurana. The requisite genes include the translation initiation factors EIF4G, whose role in PD is disputed, and ATNX2, implicated in ataxia as well as ALS risk, and several ribosomal subunits. In further support of this, the researchers found defective protein synthesis in HEK cells overexpressing α-synuclein and in iPSC-derived neurons harboring α-synuclein with the A53T mutation. These translation problems occurred before the canonical ER stress response had a chance to kick in, Khurana said. Overexpressing EIF4G1 or ATXN2 reversed these protein translation defects. The researchers hypothesize that the involvement of so many factors related to mRNA processing and translation implies a deep biological connection between α-synuclein and these processes.
Touched by α-Synuclein
In their second paper, Chung and Khurana, as co-first and corresponding author, provided support for these and other associations discovered in the genetic study. Using a technique called ascorbate peroxidase (APEX) labeling, which was developed by Alice Ting and colleagues at Massachusetts Institute of Technology, the researchers identified 225 proteins in close physical proximity to α-synuclein in neurons. Older methods such as co-immunoprecipitation require lysing of the cell and tend to identify only stable protein complexes; in contrast, APEX identifies even transient protein-protein interactions while the cells still live. The researchers employed the technique by fusing α-synuclein with APEX, which oxidizes phenol derivatives to phenol radicals. These extremely short-lived radicals then covalently react with amino acids in their immediate vicinity, allowing identification of labeled proteins by mass spectrometry.
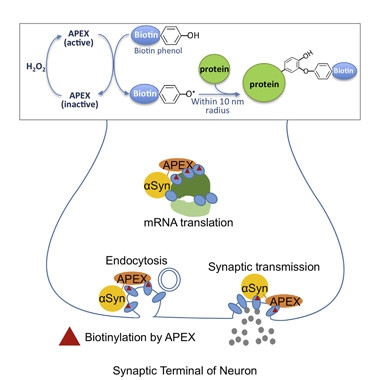
Social Synuclein.
The APEX enzyme, fused to α-synuclein, labels nearby proteins in neurons. Proteins involved in endocytosis, synaptic transmission, and mRNA translation were targets. [Courtesy of Chung et al., Cell System 2017.]
Chung and colleagues transduced rat primary cortical neurons with α-synuclein fused to APEX2, a catalytically superior version of APEX, and then ran mass spec to unveil α-synuclein’s social network. Many of the 225 proteins the researchers uncovered hailed from α-synuclein’s known stomping grounds of vesicles and synaptic terminals. Biological processes that had been well represented in the genetic networks, such as protein transport and vesicular trafficking, once again made a showing in the APEX assay. In addition, microtubule-associated proteins, including tau, rubbed shoulders with α-synuclein, as did proteins involved with mRNA binding, processing, and translation.
Khurana was particularly intrigued by this last group of interactions. “Synuclein is physically and genetically interacting with translation and mRNA metabolism factors in a way that is surprising and distinct from other neurodegenerative disease proteins,” he told Alzforum. “The two papers together would suggest that there may be a physiological role for α-synuclein in mRNA metabolism that was not previously appreciated.” He speculated that perhaps α-synuclein physically associates with translation factors and sequesters them. This could occur in the synapse, where synuclein concentrates and local mRNA translation plays a key role in synaptic plasticity.
Mark Cookson of the National Institutes of Health in Bethesda, Maryland, was not surprised that translation appears important in α-synuclein toxicity. He noted two recent studies implicating the PD genes PINK, Parkin, and LRRK2 in mRNA translation (see Gehrke et al., 2015; Apr 2014 news). Still, Cookson favors the idea that α-synuclein and translation are most strongly linked via malfunctions in vesicle trafficking, which halt translation.
Because the APEX2 map was generated in neurons, it captured neuronal-specific interactions that the humanized yeast genetic screens missed. The spatial map featured multiple neuron-specific RAB3 proteins involved in synaptic vesicle exocytosis. Previous studies had implicated these proteins as modifiers of α-synuclein toxicity in neurons (see Gitler et al., 2008; Chung et al., 2009). The researchers went on to confirm many of the physical interactions identified in the APEX2 screen using a technique called membrane yeast two-hybrid. MYTH is a spin-off of classical yeast two-hybrid methods; it works well for α-synuclein, which is primarily a membrane-associated protein.
Khurana thinks it is reassuring that a number of the genes and proteins had known ties to PD. “Visualizing how they fit together is important and makes one wonder how many of the other genes in our network are related to PD risk,” Khurana said. Two of the genes in the network, calcineurin and Nedd4, are targets of available drugs that reduced α-synuclein pathology in mouse models, and he suggested the network approach might one day lead to the development of therapies aimed at mechanisms driving specific forms of disease (see Oct 2013 news and Aug 2014 news). Cookson especially welcomed the integration of parkinsonism genes into the APEX2 network. Should treatments beyond dopaminergic therapies pan out someday, it could help steer patients toward therapies specifically tailored to address the biological mechanisms that underlie their disease, he said. On the flip side, the networks also point out that patients with different clinical presentations may share common mechanisms of disease, such as defects in vesicular trafficking, he added.
In their tribute to Lindquist, Khurana, Chung, and Tardiff wrote that they believe in the potential of yeast-to-human approaches, but were careful not to tout them too heavily until they yield effective therapies. The three researchers co-founded Yumanity Therapeutics with Lindquist to translate findings in yeast and human neurons into therapies for neurodegenerative disease. “We continue to fight the good fight, spurred on by memories of a transformative woman and the uncommonly beautiful biology she brought back from a billion years ago,” they wrote.—Jessica Shugart
References
News Citations
- Largest Meta-GWAS Yet Uncovers New Genetic Links to Parkinson’s
- Yeast Teases Apart Huntington’s and Parkinson’s Protein Aggregation
- Traffic Control—Curb Endocytosis to Curb AD Pathogenesis?
- Heady Times for Researchers Studying TDP-43
- Yeast May Point the Way to Effective Parkinson’s Drugs
- Stress Relief: Anti-Stress Granule Therapy Saves ALS Models
- Protecting Neurons by Ramping Up Waste Disposal?
- ALS—A Polyglutamine Disease? Mid-length Repeats Boost Risk
- Another Partner for LRRK2: Will This Relationship Endure?
- Could Toning Down Calcineurin Neutralize α-Synuclein?
Paper Citations
- Treusch S, Hamamichi S, Goodman JL, Matlack KE, Chung CY, Baru V, Shulman JM, Parrado A, Bevis BJ, Valastyan JS, Han H, Lindhagen-Persson M, Reiman EM, Evans DA, Bennett DA, Olofsson A, DeJager PL, Tanzi RE, Caldwell KA, Caldwell GA, Lindquist S. Functional links between Aβ toxicity, endocytic trafficking, and Alzheimer's disease risk factors in yeast. Science. 2011 Dec 2;334(6060):1241-5. PubMed.
- Yeger-Lotem E, Riva L, Su LJ, Gitler AD, Cashikar AG, King OD, Auluck PK, Geddie ML, Valastyan JS, Karger DR, Lindquist S, Fraenkel E. Bridging high-throughput genetic and transcriptional data reveals cellular responses to alpha-synuclein toxicity. Nat Genet. 2009 Mar;41(3):316-23. Epub 2009 Feb 22 PubMed.
- Gehrke S, Wu Z, Klinkenberg M, Sun Y, Auburger G, Guo S, Lu B. PINK1 and Parkin control localized translation of respiratory chain component mRNAs on mitochondria outer membrane. Cell Metab. 2015 Jan 6;21(1):95-108. PubMed.
- Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, Barlowe C, Lindquist S. The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci U S A. 2008 Jan 8;105(1):145-50. PubMed.
- Chung CY, Koprich JB, Hallett PJ, Isacson O. Functional enhancement and protection of dopaminergic terminals by RAB3B overexpression. Proc Natl Acad Sci U S A. 2009 Dec 29;106(52):22474-9. PubMed.
External Citations
Further Reading
Papers
- Khurana V, Lindquist S. Modelling neurodegeneration in Saccharomyces cerevisiae: why cook with baker's yeast?. Nat Rev Neurosci. 2010 Jun;11(6):436-49. PubMed.
External Links
Primary Papers
- Khurana V, Peng J, Chung CY, Auluck PK, Fanning S, Tardiff DF, Bartels T, Koeva M, Eichhorn SW, Benyamini H, Lou Y, Nutter-Upham A, Baru V, Freyzon Y, Tuncbag N, Costanzo M, San Luis BJ, Schöndorf DC, Barrasa MI, Ehsani S, Sanjana N, Zhong Q, Gasser T, Bartel DP, Vidal M, Deleidi M, Boone C, Fraenkel E, Berger B, Lindquist S. Genome-Scale Networks Link Neurodegenerative Disease Genes to α-Synuclein through Specific Molecular Pathways. Cell Syst. 2017 Feb 22;4(2):157-170.e14. Epub 2017 Jan 25 PubMed.
- Chung CY, Khurana V, Yi S, Sahni N, Loh KH, Auluck PK, Baru V, Udeshi ND, Freyzon Y, Carr SA, Hill DE, Vidal M, Ting AY, Lindquist S. In Situ Peroxidase Labeling and Mass-Spectrometry Connects Alpha-Synuclein Directly to Endocytic Trafficking and mRNA Metabolism in Neurons. Cell Syst. 2017 Feb 22;4(2):242-250.e4. Epub 2017 Jan 25 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Columbia University
Brandeis University
This duet of papers represents the swan song of one of the deepest thinkers into the fundamental biology of neurodegeneration, our recently departed friend Sue Lindquist, who sadly died a few months ago. The papers illustrate the magnificent creativeness and scope of her life’s work, integrating yeast, genomic, and computational biology with symphonic elegance.
In previous papers, Sue and her team had used such an integrative approach to identify molecular networks linked to individual proteinopathies, Aβ toxicity associated with Alzheimer’s (Treusch et al., 2011), and α-synuclein toxicity associated with Parkinson’s disease (Chung et al., 2013). Sue always believed that there must be mechanistic themes that overlie and unify these proteinopathies, and in fact, AD and PD are associated disorders that are converging more and more, in their histological and molecular makers, and ultimately in their clinical presentations. While the primary focus in the current papers is on α-synuclein, and they introduce a plethora of findings, we know she was especially pleased that they essentially confirmed her unfailing intuition: namely, that the proteinopathies observed in many neurodegenerative diseases appear driven by overlapping mechanistic pathways, especially at the level of cell biology. The two top pathways that emerge from these two papers as fundamental drivers of neurodegenerative diseases are endocytic trafficking and mRNA metabolism. More specifically, and what unsurprisingly is most interesting to us (Small and Petsko, 2015), retromer-related molecules were the dominant unifiers in the endocytic trafficking pathway, including retromer’s molecular core protein Vps35 (see especially Figure 3B in the second paper). While the entire multimodular retromer assembly seems to be a molecular point of convergence in PD and AD, genetic studies are beginning to explain how subtle biochemical differences in defects observed in the single molecule Vps35 can be linked more with one than the other (Rovelet-Lecrux et al., 2015). The systems biology approach taken by Sue Lindquist in these papers represents the latest, and certainly one of the most elegant, set of evidence in a rapidly solidifying case that endosomal trafficking is a cell biology pathway at the heart of the major neurodegenerative diseases.
Make a Comment
To make a comment you must login or register.