FTD-GRN: A Disease of Angiogenesis and Vascular Fibrosis?
Quick Links
Mutations in the progranulin gene (GRN) cause autosomal-dominant frontotemporal dementia, but how cells go awry in this disease is unclear. In the first single-nucleus RNA-sequencing study of FTD, researchers led by Bart Eggen, University of Groningen, Netherlands, and John van Swieten, Erasmus University Medical Center, Rotterdam, uncovered changes in astrocytes and neurovascular cells. In the July 25 Nature Neuroscience, they reported that astrocytes became reactive, fibroblasts more numerous, and endothelial cells and pericytes were depleted. Signaling pathways involved in BBB dysfunction and angiogenesis ramped up, as did fibrotic, hypertrophied blood vessels. Strangely, perhaps, microglia were unscathed.
- First snRNA-Seq of FTD-GRN shows aberrant signaling pathways involved with BBB and angiogenesis.
- Cortical tissue contains fibrotic, swollen blood vessels.
- Microglia? Unperturbed.
“These types of unbiased studies steer researchers in interesting and surprising ways,” Andrew Yang, University of California, San Francisco, told Alzforum. “It is striking to see strong perturbations of vascular cells and not microglia in the context of severe neuronal loss at end-stage disease.”
Yang believes the findings shift people’s preconceptions of which cell types are the most important for the pathogenesis of FTD. Deepti Lall, Cedars-Sinai, Beverly Hills, California, agreed. “These critical findings implicate an understudied and underappreciated yet major role of BBB and neurovascular dysfunction in FTD,” she wrote (full comment below).
Progranulin is a growth factor that supports angiogenesis, inflammation, and brain development (Eguchi et al., 2017). It plays an important role in lysosomal function (reviewed by Kao et al., 2017). People with FTD-GRN, who carry one mutant progranulin allele, have high plasma and cerebrospinal fluid levels of reactive astrocyte markers, such as glial fibrillary acidic protein (GFAP), or pro-inflammatory cytokines such as TNF-α and interleukins (Heller et al., 2020; Bossù et al., 2011). Microglia were assumed to be likely culprits of this response. That said, little is known about what happens to brain cells in this disease, especially glial and vascular ones, partly because the disease is so rare that postmortem tissue is hard to come by.
First author Emma Gerrits and co-workers did get their hands on such tissue. They analyzed frontal, temporal, and occipital cortex samples from 13 people who had had FTD-GRN and from seven sex- and age-matched controls from the Netherlands Brain Bank and the French Neuro-CEB brain bank. After isolating nuclei, she used fluorescence-activated sorting to separate NEUN-positive neuronal nuclei and OLIG2-positive oligodendrocyte nuclei from all other types. “This allowed us to obtain a huge dataset of microglia, astrocytes, and neurovascular cells, which gave us a lot of statistical power to identify changes,” Gerrits told Alzforum.
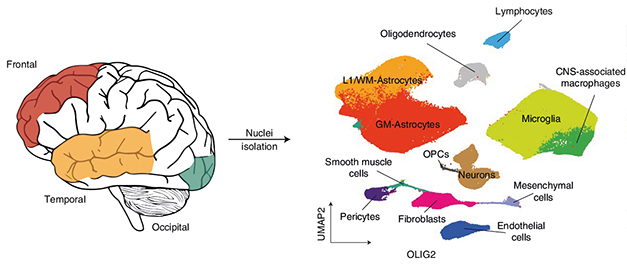
Sorting Game. Single nucleus RNA-Seq of frontal, temporal, and occipital cortex (left) identified different cell types associated with FTD-GRN (right). [Courtesy of Gerrits et al., Nature Neuroscience, 2022.]
RNA-Seq analysis clustered 432,000 of those cells into subtypes, of which astrocytes and microglia were the most abundant (see image above). Though control and FTD samples had similar proportions of astrocytes, the cells were transcriptionally distinct. FTD tissue had more of four subgroups of astrocytes expressing genes involved in wound healing, fluid transport, interferon signaling, and response to oxidative stress.
Three upregulated genes caught the researchers’ eye: GFAP, a marker highly expressed in reactive astrocytes; AQP4, the water channel on astrocytic end feet that surround blood vessels and are important for the blood-brain barrier to function; and WDR49, which encodes a protein of unknown function. Immunohistochemistry of FTD frontal cortex tissue detected stronger GFAP and AQP4 labeling, too.
WDR49 intrigued the scientists because its distribution mirrored the progression of neurodegeneration seen in FTD, from the frontal to the temporal cortices. Immunohistochemistry detected clusters of WDR49- and GFAP-positive cells in all of the frontal and half of the temporal cortex samples, yet in none of the occipital cortices, which are spared in this disease. “The clumps were scattered all over the cortex with no link to TDP-43 accumulation or to blood vessels,” Gerrits said. However, WDR49-positive astrocytes did correlate with neurodegeneration.
The authors believe WDR49 might be a new neuropathological marker for FTD-GRN because they found no upregulation in Alzheimer’s disease tissue (Gerrits et al., 2021). Gerrits is correlating immunohistochemical with spatial transcriptomic analyses to determine if WDR49 clusters correlate with any specific pathology.
Intriguingly, in the FTD-GRN brain microglia were as abundant and similarly distributed as in controls. There was no difference in the numbers of stress-associated, pro-inflammatory, or capillary-associated microglia. This absence of microgliosis corroborates previous findings in heterozygous GRN knockout mice (Filiano et al., 2013; Arrant et al., 2019). “Microglial-driven neuroinflammation is not a key factor in FTD-GRN,” the authors concluded.
Neurovascular dysfunction may be, however. Compared to controls, FTD-GRN tissue had fewer endothelial cells and pericytes but more fibroblasts, smooth muscle, and mesenchymal cells. Subgroup analysis painted a complicated picture. Endothelial cells expressing the tight junction protein CLDN5 were depleted in FTD, while those expressing genes linked to angiogenesis and hypervascularization were more numerous. The most abundant fibroblasts expressed genes encoding collagen and other extracellular matrix (ECM) proteins.
What might this have done to blood vessels? To investigate, the scientists used immunofluorescence of CLDN5 and fibronectin, an ECM protein that shores up weakened blood vessel walls to aid in healing. Too much fibronectin causes vessel fibrosis. Lo and behold, Gerrits detected swollen vessels covered by fibronectin, which had few CLDN5-positive endothelial cells. The vessels formed clusters similar to those recently reported to be indicative of abnormal angiogenesis in FTD, vascular dementia, and Alzheimer’s disease (Olofsson et al., 2019). The authors hypothesized that diseased vasculature was permeable due to a weak layer of endothelial cells, which induced neuroinflammation, triggered fibrosis, and spurred new vessel formation to compensate for vascular breakdown.

Fibrotic Vessel. A blood vessel in FTD frontal cortex tissue has a weak endothelial lining (CLDN5, teal) and fibrosis on its outside (fibronectin, pink). [Courtesy of Gerrits et al., Nature Neuroscience, 2022.]
In FTD-GRN, endothelial cells expressed fewer BBB-specific genes, hence the researchers wondered if cells that support the BBB were perturbed (see Nov 2019 news). Indeed, Gerrits et al. found fewer pericytes in FTD tissue than in controls.
Beyond loss of cells, could disrupted interactions between vascular cells also damage the BBB? To test this, the scientists turned to CellChat to probe how frontal cortex cells interacted with one another. Using snRNA-Seq data, this algorithm infers intercellular signaling based on expression of ligands and receptors in different cells (Jin et al., 2021). CellChat indicated that, in FTD-GRN, endothelial cells signaled to pericytes more than they did in control tissue, but that pericytes were less receptive. The authors believe that without responses from the pericytes, endothelial cells may deteriorate, undermining the BBB.
As for other cell-cell communication, astrocytes, endothelial cells, fibroblasts, and mesenchymal cells “chatted” more with each other in FTD than control tissue, according to this analysis tool, and pathways involving vascularization, BBB function, and neurotrophic signaling were the topics of these “communiques.” Specifically, signaling of the chemokine family CXCL and the neurotrophic factor NGF stood out. The former associate with inflammation and BBB dysfunction, the latter with angiogenesis (Haarmann et al., 2019; Cantarella et al., 2002).
All told, the data suggest that neurovascular cells become dysfunctional in the frontal and temporal cortices in FTD-GRN, placing BBB breakdown at the center of its pathophysiology. “It would be interesting to investigate if similar neurovascular and BBB dysfunctions are observed in FTD patients with mutations in other genes, such as C9ORF72 and TBK1, to identify common and variant-specific disease mechanisms,” Lall said. Gerrits is already running similar snRNA-Seq analyses on tissue from people who had different subtypes of FTD.
A hint comes from amyotrophic lateral sclerosis, a related disease in which perivascular fibroblasts are reported to go berserk just before symptoms begin (Månberg et al., 2021).
Eggen thinks that neurovascular dysfunction, rather than microgliosis, may underlie neurodegeneration within the spectrum of ALS-FTD diseases.—Chelsea Weidman Burke
References
News Citations
Paper Citations
- Eguchi R, Nakano T, Wakabayashi I. Progranulin and granulin-like protein as novel VEGF-independent angiogenic factors derived from human mesothelioma cells. Oncogene. 2017 Feb 2;36(5):714-722. Epub 2016 Jun 27 PubMed.
- Kao AW, McKay A, Singh PP, Brunet A, Huang EJ. Progranulin, lysosomal regulation and neurodegenerative disease. Nat Rev Neurosci. 2017 Jun;18(6):325-333. Epub 2017 Apr 24 PubMed.
- Heller C, Foiani MS, Moore K, Convery R, Bocchetta M, Neason M, Cash DM, Thomas D, Greaves CV, Woollacott IO, Shafei R, Van Swieten JC, Moreno F, Sanchez-Valle R, Borroni B, Laforce R Jr, Masellis M, Tartaglia MC, Graff C, Galimberti D, Rowe JB, Finger E, Synofzik M, Vandenberghe R, de Mendonca A, Tagliavini F, Santana I, Ducharme S, Butler CR, Gerhard A, Levin J, Danek A, Frisoni G, Sorbi S, Otto M, Heslegrave AJ, Zetterberg H, Rohrer JD, GENFI. Plasma glial fibrillary acidic protein is raised in progranulin-associated frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2020 Mar;91(3):263-270. Epub 2020 Jan 14 PubMed.
- Bossù P, Salani F, Alberici A, Archetti S, Bellelli G, Galimberti D, Scarpini E, Spalletta G, Caltagirone C, Padovani A, Borroni B. Loss of function mutations in the progranulin gene are related to pro-inflammatory cytokine dysregulation in frontotemporal lobar degeneration patients. J Neuroinflammation. 2011;8:65. PubMed.
- Gerrits E, Brouwer N, Kooistra SM, Woodbury ME, Vermeiren Y, Lambourne M, Mulder J, Kummer M, Möller T, Biber K, Dunnen WF, De Deyn PP, Eggen BJ, Boddeke EW. Distinct amyloid-β and tau-associated microglia profiles in Alzheimer's disease. Acta Neuropathol. 2021 May;141(5):681-696. Epub 2021 Feb 20 PubMed.
- Filiano AJ, Martens LH, Young AH, Warmus BA, Zhou P, Diaz-Ramirez G, Jiao J, Zhang Z, Huang EJ, Gao FB, Farese RV, Roberson ED. Dissociation of frontotemporal dementia-related deficits and neuroinflammation in progranulin haploinsufficient mice. J Neurosci. 2013 Mar 20;33(12):5352-61. PubMed.
- Arrant AE, Filiano AJ, Patel AR, Hoffmann MQ, Boyle NR, Kashyap SN, Onyilo VC, Young AH, Roberson ED. Reduction of microglial progranulin does not exacerbate pathology or behavioral deficits in neuronal progranulin-insufficient mice. Neurobiol Dis. 2019 Apr;124:152-162. Epub 2018 Nov 15 PubMed.
- Ek Olofsson H, Englund E. A cortical microvascular structure in vascular dementia, Alzheimer's disease, frontotemporal lobar degeneration and nondemented controls: a sign of angiogenesis due to brain ischaemia?. Neuropathol Appl Neurobiol. 2019 Oct;45(6):557-569. Epub 2019 May 9 PubMed.
- Jin S, Guerrero-Juarez CF, Zhang L, Chang I, Ramos R, Kuan CH, Myung P, Plikus MV, Nie Q. Inference and analysis of cell-cell communication using CellChat. Nat Commun. 2021 Feb 17;12(1):1088. PubMed.
- Haarmann A, Schuhmann MK, Silwedel C, Monoranu CM, Stoll G, Buttmann M. Human Brain Endothelial CXCR2 is Inflammation-Inducible and Mediates CXCL5- and CXCL8-Triggered Paraendothelial Barrier Breakdown. Int J Mol Sci. 2019 Jan 30;20(3) PubMed.
- Cantarella G, Lempereur L, Presta M, Ribatti D, Lombardo G, Lazarovici P, Zappalà G, Pafumi C, Bernardini R. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002 Aug;16(10):1307-9. Epub 2002 Jun 21 PubMed.
- Månberg A, Skene N, Sanders F, Trusohamn M, Remnestål J, Szczepińska A, Aksoylu IS, Lönnerberg P, Ebarasi L, Wouters S, Lehmann M, Olofsson J, von Gohren Antequera I, Domaniku A, De Schaepdryver M, De Vocht J, Poesen K, Uhlén M, Anink J, Mijnsbergen C, Vergunst-Bosch H, Hübers A, Kläppe U, Rodriguez-Vieitez E, Gilthorpe JD, Hedlund E, Harris RA, Aronica E, Van Damme P, Ludolph A, Veldink J, Ingre C, Nilsson P, Lewandowski SA. Altered perivascular fibroblast activity precedes ALS disease onset. Nat Med. 2021 Apr;27(4):640-646. Epub 2021 Apr 15 PubMed. Correction.
External Citations
Further Reading
Primary Papers
- Gerrits E, Giannini LA, Brouwer N, Melhem S, Seilhean D, Le Ber I, Brainbank Neuro-CEB Neuropathology Network, Kamermans A, Kooij G, de Vries HE, Boddeke EW, Seelaar H, van Swieten JC, Eggen BJ. Neurovascular dysfunction in GRN-associated frontotemporal dementia identified by single-nucleus RNA sequencing of human cerebral cortex. Nat Neurosci. 2022 Aug;25(8):1034-1048. Epub 2022 Jul 25 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Cedars-Sinai
FTD is a complex neurodegenerative disorder characterized by neuronal loss in frontal and temporal lobes leading to loss of cognitive, social, and emotional abilities. Heterozygous mutations in progranulin (GRN) generally result in reduced protein levels of progranulin and are among the most common genetic causes of FTD (FTD-GRN). Despite recent advancements in understanding of FTD-GRN disease mechanisms, the relative contribution of different cell types, particularly glial and vascular cells, to pathogenesis is largely unresolved. Additionally, lack of profiling of postmortem tissue from FTD-GRN patients necessitates efforts to fully understand the FTD-GRN pathophysiology.
This manuscript is an important step in this direction because it shows, for the first time, the cellular dysfunction of these “supporting cells” in the brains of FTD-GRN patients. Using single nuclear sequencing from the nuclei isolated from affected (frontal and temporal) and unaffected (occipital) lobes from gray matter of FTD-GRN postmortem tissue, the authors show neurovascular and blood-brain barrier dysfunction in these patients. Apart from cortical degeneration, the brains of these patients show vascular fibrosis and altered expression of genes associated with neurovascular cell-cell interactions, and with astrocyte and pericyte function. Surprisingly, the resident immune cells of the brain, microglia, which had been previously implicated in GRN-disease pathogenesis, were only mildly affected.
These critical findings implicate an understudied, underappreciated, but major role of blood-brain barrier and neurovascular dysfunction in FTD. Though neurodegenerative disorders are predominately characterized by neuronal loss that causes their symptoms, the role played by other brain-cell types in disease pathogenesis requires more attention. This study enhances our understanding of the role of such cells in FTD pathophysiology. BBB dysfunction is important because impaired transport of metabolites across the BBB and associated neurovascular dysfunction can have deleterious effects on neuronal health.
The lack of neuroinflammation in FTD-GRN patients came as a surprise, since progranulin gene expression is highest in microglia and studies in mouse models have shown that these cells play a critical role in FTD disease pathogenesis. However, since most studies were done on GRN knockout mice, the mechanisms underlying microgliosis and neuronal loss in mice can be completely different than those observed in humans.
Moving forward, it would be interesting to investigate if similar neurovascular and BBB dysfunctions are observed in FTD patients with mutations in other genes, such as C9ORF72, TBK1, etc., to identify common and variant disease mechanisms. Additionally, it will be highly desirable to model these dysfunctions in patient-derived iPSC cells that can be used to identify early disease mechanisms and test different therapeutics for intervention.
Make a Comment
To make a comment you must login or register.