First Crystal Structure of a Presenilin
Quick Links
In this week’s Nature, researchers report the first successful crystal structure of a bacterial presenilin homologue. The human presenilin forms the active part of the transmembrane γ-secretase complex that releases Aβ from its precursor, but it has resisted previous attempts at crystallization and detailed structural study. The authors, led by Yigong Shi at Tsinghua University, Beijing, China, got around that problem by instead using a closely related archaebacterial presenilin/SPP homologue (PSH). Their structural findings largely agree with previous electron microscopy studies of γ-secretase (see ARF related news story and ARF news story). They include more detail and also contain a few surprises, noted Michael Wolfe at Brigham and Women’s Hospital, Boston, Massachusetts, in an accompanying Nature commentary. The biggest surprise is that a small pore pierces through the entire transmembrane region of the protein, raising the question of whether ions or water could pass through the complex.
Christian Haass at Ludwig-Maximilians University, Munich, Germany, wrote to Alzforum that the work represents a “…fantastic breakthrough. I am extremely pleased that many of the seminal findings on structure/function relationships made by numerous researchers in the field were fully confirmed.” (See full comment below.)
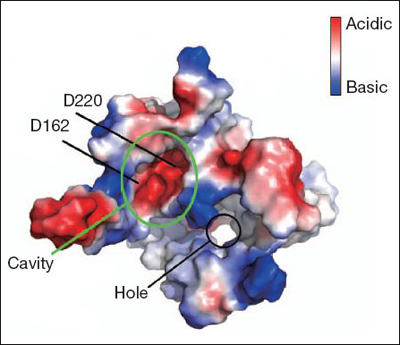
The crystal structure confirms that presenilin has nine transmembrane domains (TMDs). Two aspartate residues critical for enzymatic cleavage lie in TMD6 and 7, inside the cell membrane but facing a cytoplasmic pocket that would give them access to water. This is crucial because presenilin needs water to hydrolyze its substrates. Also consistent with previous work (see Kornilova et al., 2005; Sato et al., 2008), the data suggest that substrates slide into presenilin through an open space between TMD6 and TMD9, which exposes them to the active site and its watery cavity. The unexpected finding was the presence of a membrane-spanning hole nestled between TMD2, 3, 5, and 7 (see image below). This pore could provide an additional way for water to reach the active site, or for ions to pass through the protein, Wolfe suggested in his commentary. Some researchers have proposed that presenilin acts as a calcium channel (see ARF related news story). Alternatively, the hole might be plugged by lipids or other small molecules in vivo, the authors note.
Model of PSH shows watery cavity at the active site and a transmembrane pore. Image courtesy of Yigong Shi and Nature
“[The authors’] work opens up a whole new horizon that should ultimately lead to a detailed understanding of human presenilins and of the entire γ-secretase complex. Such understanding should lead to specific ideas about how disease-causing mutations alter function, and how small molecules might be designed for safe and effective treatment of Alzheimer’s disease,” Wolfe wrote.––Madolyn Bowman Rogers
References
News Citations
- Divide and Conquer: Structure-Function Victory With Presenilin 1
- Keystone: γ Slowly Relinquishes Its Secrets
- Presenilins Open Escape Hatch for ER Calcium
Paper Citations
- Kornilova AY, Bihel F, Das C, Wolfe MS. The initial substrate-binding site of gamma-secretase is located on presenilin near the active site. Proc Natl Acad Sci U S A. 2005 Mar 1;102(9):3230-5. PubMed.
- Sato C, Takagi S, Tomita T, Iwatsubo T. The C-terminal PAL motif and transmembrane domain 9 of presenilin 1 are involved in the formation of the catalytic pore of the gamma-secretase. J Neurosci. 2008 Jun 11;28(24):6264-71. PubMed.
Other Citations
Further Reading
Primary Papers
- Wolfe MS. Structural biology: Membrane enzyme cuts a fine figure. Nature. 2013 Jan 3;493(7430):34-5. PubMed.
- Li X, Dang S, Yan C, Gong X, Wang J, Shi Y. Structure of a presenilin family intramembrane aspartate protease. Nature. 2013 Jan 3;493(7430):56-61. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Brookhaven National Laboratory
This is a very important and extremely interesting work. I am glad to see the 8-angstrom-deep water-accessible cavity near the cytosolic side of the membrane. We saw this in our cryo-EM structure in 2008. We suspected, but didn't have evidence at that time, that this is where the active site is. The transmembrane pore is mysterious. It is hydrophobic, so water or ions are unlikely to pass through.
This pore could be plugged in vivo either by lipid or other partner proteins. Alternatively, the pore may function/facilitate in accommodating single transmembrane helical substrates.
Biomedizinisches Centrum (BMC), Biochemie & Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE)
This is certainly an absolutely fantastic breakthrough. For the very first time, we can see structural details of presenilin! Moreover, I am extremely pleased that many of the seminal findings on structure/function relationships made by numerous researchers in the field were fully confirmed. I find it very interesting that Li et al. crystallized an apparently inactive protease. Maybe that explains what we and others observed upon pull-down of γ-secretase with biotinylated inhibitors.
Apparently, we can capture only a minority of γ-secretase, whereas a large portion remains (inactive?) in the supernatant. Obviously, such great findings immediately ask for more details: I would, for example, love to see a co-crystal with a γ-secretase inhibitor or a γ-secretase substrate. Finally, I think PSH is more an SPP/SPPL-like protease. To prove that, the membrane orientation should be determined.
But these are just additional questions, which now, based on the pioneering work of Li et al., can be addressed.
KU Leuven
The crystal structure of an archaea homologue of presenilin (mmPSH), just published in Nature, represents a substantial advance in the structural field of membrane proteins that could shed light on the structural basis of the active site of the γ-secretase complex.
The authors carried out a tremendous amount of work to identify well-expressing and stable presenilin homologues that enhance crystallizability. In fact, the report illustrates the enormous efforts and creativity needed to achieve such structural investigation.
Importantly, the crystal structure is consistent with the 9 transmembrane domain (TMD) organization proposed for presenilin, and confirms that the catalytic site is located in a cavity connected to the cytoplasmic side, illustrating how water molecules reach the catalytic aspartates. However, the mmPSH structure seems to represent an inactive state of the protease, in which the catalytic aspartates are uncoupled, indicating that structural adjustments should occur in order to bring it into the active conformation. Interestingly, this observation is common to the recently reported structure of the GXGD protease FlaK (Hu et al., 2011), and may represent a universal mechanism regulating intramembrane catalysis.
In the absence of an atomic structure of presenilin, the mmPSH crystal structure provides a structural framework to model presenilin structure. Actually, Li et al. modeled human presenilin 3D-structure using the mmPSH crystal structure as a template. Based on this model, they propose a potential route of entry for substrates (between TMD6 and TMD9), which seems to be consistent with reported experimental data. Li et al. propose that binding of substrates triggers structural rearrangements in mmPSH that result in an active state. However, this hypothesis needs validation, since the reported structure may (just) represent a stable (inactive) conformation that differs from the “active and ready to bind substrate” presenilin conformation. In fact, the active presenilin conformation likely depends on interactions with other γ-secretase subunits, for instance, Pen-2 (Ahn et al., 2010).
Along the same reasoning, although the homologue protease seems to share the same overall structure with presenilin, there are some limitations on the functional side. Clearly, the analysis performed on mmPSH mutations that are analogous to FAD-causing mutations in human presenilin 1 does not provide relevant information on the effect/mechanism of the disease-causing mutations. The limitation likely emerges from the fact that these kinds of activity assays only assess the overall activity of the mmPSH and, importantly, FAD-linked human presenilin mutations may not have an effect on the “overall” activity but specifically at the production of Aβ peptides from the APP substrate (Chávez-Gutiérrez et al., 2012). This actually raises an interesting point: whether the mmPSH processes the test substrate Gurken (or other substrates) in the way presenilin does, i.e., processive proteolytic cuts from ε-endoprotease to γ-carboxypeptidase-like cleavages (Takami et al., 2009).
Despite the limitations, the mmPSH structure will allow us to apply, for the first time, rational approaches to understand presenilin function, inactivation, and modulation, which may boost drug discovery for Alzheimer’s and cancer. Furthermore, this report encourages structural studies on membrane proteases, in particular, γ-secretase.
References:
Hu J, Xue Y, Lee S, Ha Y. The crystal structure of GXGD membrane protease FlaK. Nature. 2011 Jul 28;475(7357):528-31. PubMed.
Ahn K, Shelton CC, Tian Y, Zhang X, Gilchrist ML, Sisodia SS, Li YM. Activation and intrinsic gamma-secretase activity of presenilin 1. Proc Natl Acad Sci U S A. 2010 Dec 14;107(50):21435-40. PubMed.
Chávez-Gutiérrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M, Lismont S, Zhou L, Van Cleynenbreugel S, Esselmann H, Wiltfang J, Serneels L, Karran E, Gijsen H, Schymkowitz J, Rousseau F, Broersen K, De Strooper B. The mechanism of γ-Secretase dysfunction in familial Alzheimer disease. EMBO J. 2012 May 16;31(10):2261-74. Epub 2012 Apr 13 PubMed.
Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y. gamma-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci. 2009 Oct 14;29(41):13042-52. PubMed.
The University of Tokyo
By Taisuke Tomita and Takeshi Iwatsubo
Li and colleagues have provided the first crystal structure of an archaeal presenilin protein. As the authors mentioned, despite extensive years of efforts on eukaryotic presenilins, the crystal structure of PS has not been elucidated. The findings of Li et al. are significant in that they obtained crystals of “proteolytically active” forms of presenilin/SPP homologue (PSH) that are suitable for X-ray diffraction analysis, by testing a series of mutants as well as adopting protease digestion. Taken together with the previous structural data on rhomboid and S2P, this new PSH structure strongly supports the notion that “intra”membrane cleavage is a general proteolytic reaction occurring in the hydrophilic environment within the lipid bilayer.
The structure exhibits several intriguing features: Notably, the catalytic module of PSH is formed by transmembrane domains (TMD) 6-9, and TMD9 is likely to serve as a gate for substrate entry. This dovetails quite well with the predicted structural features based on cysteine scanning mutagenesis reported by ourselves and by other researchers (Sato et al., 2006; Sato et al., 2008; Tolia et al., 2008). Thus, the TMDs 1-5 support the structure of the catalytic cavity laden with the mysterious "hole." These TMDs appear to form interfaces within the tetrameric assembly of PSH that was identified in the crystal. Interestingly, this mode of assembly is quite reminiscent to one that we observed in the single particle analysis of SPP (Miyashita et al., 2011). The next goal for these lines of structural studies would be to elucidate the structure of PSH bound to a transition state analogue, which should unveil the mode of substrate recognition at the catalytic site. The structure of the active form of γ-secretase complex, which comprises four integral membrane protein subunits (i.e., PS, NCT, APH-1, and PEN-2), still remains unclear. Nevertheless, the results of this study are highly intriguing and provide mechanistic insights into the intramembrane cleavage by presenilin family proteins.
References:
Sato C, Morohashi Y, Tomita T, Iwatsubo T. Structure of the catalytic pore of gamma-secretase probed by the accessibility of substituted cysteines. J Neurosci. 2006 Nov 15;26(46):12081-8. PubMed.
Sato C, Takagi S, Tomita T, Iwatsubo T. The C-terminal PAL motif and transmembrane domain 9 of presenilin 1 are involved in the formation of the catalytic pore of the gamma-secretase. J Neurosci. 2008 Jun 11;28(24):6264-71. PubMed.
Tolia A, Horré K, De Strooper B. Transmembrane domain 9 of presenilin determines the dynamic conformation of the catalytic site of gamma-secretase. J Biol Chem. 2008 Jul 11;283(28):19793-803. PubMed.
Miyashita H, Maruyama Y, Isshiki H, Osawa S, Ogura T, Mio K, Sato C, Tomita T, Iwatsubo T. Three-dimensional structure of the signal peptide peptidase. J Biol Chem. 2011 Jul 22;286(29):26188-97. PubMed.
UT Southwestern Medical Center at Dallas
The paper by Li et al. is a real tour de force that offers the first atomic resolution information about the three-dimensional structure of presenilins. Multiple laboratories around the world attempted to crystallize presenilins previously but have not been successful. The group headed by Yigong Shi achieved this difficult goal by focusing on an archaeal homologue called PSH, generating a series of PSH point mutants to achieve diffraction-quality crystals. As a result of this tremendous effort, the authors determined the structure of PSH at 3.3 angstrom resolution, sufficient to see most critical aspects of its conformation.
They determined that PSH is composed of nine transmembrane domains (TMDs), consistent with most recent biochemical structure-function analyses. The topology of PSH differs from two previously crystallized intramembrane proteases—rhomboid and S2 protease. The resolution of the structure is sufficiently high to visualize a large, water-filled hole that traverses the entire protein across the lipid bilayer. The hole is surrounded by TMD2, TMD3, TMD5, and TMD7. The authors state that this hole is large enough to allow passage of small ions, suggesting that PSH may function as an ion channel. In the previous studies we proposed that presenilin functions as a calcium-conducting leak channel in the endoplasmic reticulum, an activity disrupted by many familial AD mutations (1). This hypothesis has been directly challenged (2), with the argument that presenilins do not have an ion channel pore. Our previous mutagenesis data suggested that the ion-conducting pore of presenilins is lined up by residues of TMD7, but not TMD6 (3), consistent with the structure of PSH. Although future work obviously will be needed, the water-filled cavity in the PSH structure is the most likely candidate for the calcium-conducting pore in the leak channel that we proposed.
Li et al. also determined that PSH forms a tetramer in the crystals. The γ-secretase activity is likely to be supported by monomeric form of presenilins. However, many ion channels function as tetramers, and in our previous studies we determined that mutant forms of presenilin exert dominant negative effects on channel function (1). It is feasible that tetramerization of presenilins is necessary to support calcium leak function, which may explain dominant negative effects observed in our studies. This prediction also needs to be tested experimentally.
References:
Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006 Sep 8;126(5):981-93. PubMed.
Shilling D, Mak DO, Kang DE, Foskett JK. Lack of evidence for presenilins as endoplasmic reticulum Ca2+ leak channels. J Biol Chem. 2012 Mar 30;287(14):10933-44. PubMed.
Nelson O, Supnet C, Tolia A, Horré K, De Strooper B, Bezprozvanny I. Mutagenesis mapping of the presenilin 1 calcium leak conductance pore. J Biol Chem. 2011 Jun 24;286(25):22339-47. PubMed.
�
The existence of such a hole was previously envisioned in our article, where we explained the hypothetical role of such a pore.
References:
Rodríguez-Manotas M, Amorín-Díaz M, Cabezas-Herrera J, Acedo-Martínez A, Llorca-Escuín I. Are γ-secretase and its associated Alzheimer's disease γ problems?. Med Hypotheses. 2012 Feb;78(2):299-304. PubMed.
Make a Comment
To make a comment you must login or register.