Exosomes and Antibodies Tie Plasma Aβ and Tau to Alzheimer’s
Quick Links
This story was updated on 16 December 2016 to add a paragraph about exosomes in Down’s syndrome.
While changes in cerebrospinal fluid Aβ and tau reliably flag the onset of Alzheimer’s disease, plasma levels of these proteins have remained frustratingly uninformative. At the annual Society for Neuroscience conference, held November 12-16 in San Diego, speakers once again wrestled with the question of what these plasma proteins reflect, and how to detect the forms most related to pathology.
Researchers in Indiana reported that CSF and plasma tau, both of which rise in AD, each correlated with atrophy of a different set of brain regions. Meanwhile, other speakers presented approaches that home in on particular species of plasma Aβ and tau, such as oligomeric forms and those packaged in exosomes coming from the central nervous system. The neurosteroid allopregnanolone also made a showing as a potential prognostic marker. While preliminary results indicate that these various markers can pick out people on the path to AD in small samples, it remains to be seen whether any of them will prove robust.
“Progress on blood biomarkers is encouraging,” Andrew Saykin at the Indiana University School of Medicine, Indianapolis, wrote to Alzforum. “Plasma tau seems to be one of the most promising ones. The work on neuronal exosomes from peripheral blood is also exciting, and I am glad to see reports of replication.”
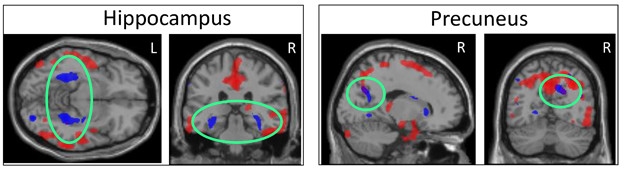
Blood and CSF Tell Different Tales. High plasma tau correlates with atrophy in specific subcortical regions (blue), while high CSF tau relates to shrinkage of largely cortical areas (red). The two patterns overlap only in precuneus (right, green circles). [Courtesy of Kacie Deters and Andrew Saykin.]
The quest for plasma biomarkers has been littered with disappointments, with many initially promising candidates failing to replicate (see Jun 2013 webinar; Feb 2016 news). A recent meta-analysis found that among the most commonly investigated plasma biomarkers, only total tau levels distinguished AD patients from controls (see Apr 2016 news). Plasma tau runs about twice as high in AD patients, but only weakly correlates with CSF total tau, leaving it unclear what these high levels signify (see Mattsson et al., 2016). One problem is that plasma tau is so low that it is difficult to measure accurately. The recent development of ultrasensitive single-molecule assays (Simoa) now allows researchers to detect quantities in the low pg/ml range or below, making measurements more reliable and opening up new frontiers for plasma biomarkers (see Apr 2016 conference news).
To find out how plasma tau relates to neurodegeneration, Kacie Deters, working with Saykin at Indiana, correlated plasma and CSF tau with brain atrophy as seen on structural MRI scans. She used data from 331 participants in ADNI-1, about one-quarter of whom were cognitively normal, half of whom had symptoms of mild cognitive impairment, and the remainder a clinical diagnosis of AD. Plasma total tau was measured with Simoa.
Deters found that a person’s plasma total tau concentration correlated largely with their degree of shrinkage in subcortical brain regions, including the parahippocampus, hippocampus, and striatum, with only a little cortical involvement in the precuneus. CSF total tau, on the other hand, correlated best with atrophy of cortical regions, particularly the medial temporal cortex. The atrophy patterns overlapped in a few brain regions, such as the precuneus, but were largely separate. Peripheral and central measures of tau may reflect different aspects of AD, or involve different tau isoforms, Deters speculated. In other studies, high plasma tau has been linked to recent brain injury and subcortical axon damage (see Mar 2014 news). Deters suggested that plasma tau could be used to screen people to find out who might need additional tests and imaging.
Other researchers are focusing on specific forms of plasma tau or Aβ. Dimitrios Kapogiannis at the National Institute on Aging, Baltimore, had previously reported that exosomes that enter blood from the central nervous system can be used to identify people with AD. Specifically, neuronally derived plasma exosomes in 57 AD patients carried more Aβ42, p-tau181, and p-tau396 than those from 57 controls, and these proteins were already elevated as early as 10 years before symptoms developed (see Aug 2014 conference news; Fiandaca et al., 2014). Because the findings came from a small cohort, the researchers are now attempting to replicate them in the Baltimore Longitudinal Study of Aging.
At SfN, Maja Mustapic in Kapogiannis’ group showed preliminary results from this cohort. She analyzed plasma samples from 350 participants, 128 of whom developed AD over the course of the study. Each participant had three samples available for analysis, spanning a four-year time period up to their diagnosis of Alzheimer’s. In neuronal exosomes isolated from the AD patients, p-tau181 and p-tau231 were higher than in controls at all time points, Mustapic reported. In contrast, total tau remained similar. The findings were highly significant and matched the data from previous studies, Kapogiannis told Alzforum. Mustapic is still analyzing Aβ42 and other markers in the exosomes. Next steps include combining Aβ and tau to develop a test that better discriminates between patients and controls, she told the audience.
Others described similar findings. In a poster, Charisse Winston, working with Robert Rissman at the University of California, San Diego, reported that exosomes from 10 AD patients and 20 people with mild cognitive impairment who progressed to AD contained more Aβ42, p-tau181, and p-tau396 than exosomes from 10 controls and 20 people with stable MCI. In addition, exosomes from people on the path to AD carried less of the synaptic protein neurogranin and the protective transcription factor REST than did those from controls (see Mar 2014 news). In particular, high p-tau181 and low neurogranin predicted progression to AD over the three years of the study, she noted. The lab will next investigate how this combination of five markers changes over time, and whether it can be used to stage AD.
In a separate experiment, the researchers injected these human exosomes into the hippocampi of young wild-type mice. One month later, levels of p-tau and aggregated tau had mushroomed in mice that received exosomes from AD patients. Exosomes from MCI patients seeded milder pathology, and those from controls, none (see Winston et al., 2016). This suggested that exosomes carry more aggregated tau as disease advances.
Plasma exosomes might also help detect AD in people with Down’s syndrome, suggested Eric Hamlett of the Medical University of South Carolina (MUSC), Charleston. Hamlett and colleagues quantified Aβ42 and p-tau in neuronally derived plasma exosomes from people with Down’s syndrome and controls across a range of ages. At SfN, Hamlett reported that neuronal exosomes from people with DS carried two to three times more Aβ42 and p-tau than those from controls, even early in life. While these markers did not correlate with cognitive status, levels of p-tau396 did. This marker shot up in people with DS who had symptoms of dementia (see Hamlett et al., 2016).
Antibodies offer a different approach to finding pathological forms of Aβ and tau in blood. Michael Sierks at Arizona State University, Tempe, previously generated several single-chain antibody fragments that identify oligomeric forms of Aβ and tau, as well as pathogenic forms of TDP-43 (see Apr 2011 conference news; Kasturirangan et al., 2010; Kasturirangan et al., 2013). These antibodies can capture femtomolar quantities of oligomers from blood and postmortem human brain samples, the researchers report (see Williams et al., 2015).
At SfN, Stephanie Williams in Sierks’ group presented preliminary results from a tiny longitudinal study of four AD patients and two age-matched controls. Their plasma samples spanned the time frame from before they developed symptoms to after their diagnoses. Before symptoms, plasma samples from the AD-bound volunteers harbored a low concentration of tau oligomers, but more pathological Aβ and TDP-43 than controls. After a diagnosis of mild cognitive impairment, tau and Aβ oligomers rose, while toxic TDP-43 fell. At the dementia stage, Aβ and tau oligomers were both high, while TDP-43 was low, Williams reported. Differences from controls were first apparent seven years before MCI developed, suggesting these markers could predict decline, she noted. Supporting this, higher levels of Aβ oligomers in the blood correlated with faster cognitive decline. The researchers are now repeating the study in a larger cohort.
What about other plasma markers? Junming Wang of University of Mississippi Medical Center, Jackson, investigated whether allopregnanolone, a metabolite of the hormone progesterone, could make a good biomarker. This hormone is in scant supply in the prefrontal and temporal cortices of Alzheimer’s patients, and its lack correlates with how severe pathology has become (see Marx et al., 2006; Naylor et al., 2010). Low allopregnanolone has been reported in the plasma of AD and Parkinson’s patients (see Smith et al., 2006; di Michele et al., 2003). Allopregnanolone is currently in a Phase 1 Alzheimer’s trial led by Roberta Brinton at the University of Southern California (see Aug 2013 conference news; Dec 2014 conference news). Wang previously worked with Brinton, but she is not involved in his current research.
To see if allopregnanolone could be a marker in AD, Wang is analyzing a cohort of 16,000 people followed at UMiss for 30 years. They donated plasma samples five times during the course of the study, starting at the beginning when everyone was still cognitively healthy. Wang and colleagues are using Simoa to measure allopregnanolone quantities down to fg/ml, he said. Preliminary data suggest this method can detect small decreases in the hormone about 10 to 15 years before cognitive symptoms appear, hinting it might be prognostic. Wang is still analyzing how well these low allopregnanolone levels predict future risk of neurodegenerative disease.—Madolyn Bowman Rogers
References
Webinar Citations
News Citations
- Replication a Challenge in Quest for Alzheimer’s Blood Test
- Meta-Analysis of 21 Years of Alzheimer's Fluid Biomarker Research
- WANTED: Biomarkers for Drug Trials in Frontotemporal Dementia
- For Hockey Players, Brain Damage Can Be Measured in Blood
- Exosomes Stand Out as Potential Blood Biomarkers
- No REST for Weary Neurons: Protective Factor Stems Cognitive Decline
- Taos: Biomarkers Keep Blooming, But Where’s the Fruit?
- Clinical Trials Roundup: Broadening the Lines of Attack
- Just for Her? Study of Women’s Biology Offers New Therapeutic Angle
Paper Citations
- Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, Palmqvist S, Baker D, Tan Hehir CA, Jeromin A, Hanlon D, Song L, Shaw LM, Trojanowski JQ, Weiner MW, Hansson O, Blennow K, ADNI Investigators. Plasma tau in Alzheimer disease. Neurology. 2016 Oct 25;87(17):1827-1835. Epub 2016 Sep 30 PubMed.
- Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2014 Aug 14; PubMed.
- Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D, Masliah E, Rissman RA. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst). 2016;3:63-72. Epub 2016 May 7 PubMed.
- Hamlett ED, Goetzl EJ, Ledreux A, Vasilevko V, Boger HA, LaRosa A, Clark D, Carroll SL, Carmona-Iragui M, Fortea J, Mufson EJ, Sabbagh M, Mohammed AH, Hartley D, Doran E, Lott IT, Granholm AC. Neuronal exosomes reveal Alzheimer's disease biomarkers in Down syndrome. Alzheimers Dement. 2016 Oct 15; PubMed.
- Kasturirangan S, Li L, Emadi S, Boddapati S, Schulz P, Sierks MR. Nanobody specific for oligomeric beta-amyloid stabilizes nontoxic form. Neurobiol Aging. 2010 Nov 8; PubMed.
- Kasturirangan S, Reasoner T, Schulz P, Boddapati S, Emadi S, Valla J, Sierks MR. Isolation and characterization of antibody fragments selective for specific protein morphologies from nanogram antigen samples. Biotechnol Prog. 2013 Mar-Apr;29(2):463-71. Epub 2013 Mar 7 PubMed.
- Williams S, Schulz P, Sierks MR. A sensitive phage-based capture ELISA for sub-femtomolar detection of protein variants directly from biological samples. Biotechnol Prog. 2015 Jan-Feb;31(1):289-98. Epub 2014 Sep 23 PubMed.
- Marx CE, Trost WT, Shampine LJ, Stevens RD, Hulette CM, Steffens DC, Ervin JF, Butterfield MI, Blazer DG, Massing MW, Lieberman JA. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer's disease. Biol Psychiatry. 2006 Dec 15;60(12):1287-94. PubMed.
- Naylor JC, Kilts JD, Hulette CM, Steffens DC, Blazer DG, Ervin JF, Strauss JL, Allen TB, Massing MW, Payne VM, Youssef NA, Shampine LJ, Marx CE. Allopregnanolone levels are reduced in temporal cortex in patients with Alzheimer's disease compared to cognitively intact control subjects. Biochim Biophys Acta. 2010 Aug;1801(8):951-9. PubMed.
- Smith CD, Wekstein DR, Markesbery WR, Frye CA. 3alpha,5alpha-THP: a potential plasma neurosteroid biomarker in Alzheimer's disease and perhaps non-Alzheimer's dementia. Psychopharmacology (Berl). 2006 Jun;186(3):481-5. PubMed.
- di Michele F, Longone P, Romeo E, Lucchetti S, Brusa L, Pierantozzi M, Bassi A, Bernardi G, Stanzione P. Decreased plasma and cerebrospinal fluid content of neuroactive steroids in Parkinson's disease. Neurol Sci. 2003 Oct;24(3):172-3. PubMed.
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.