Epigenetic Shenanigans—In AD, Chromatin Opens Up in Blood Immune Cells
Quick Links
Immune cells in the blood may be intimately involved with Alzheimer’s pathogenesis in the brain. At least, according to their chromatin. A study published January 31 in Neuron reported that in people with AD, the circulating cells—especially CD8+ T cells and monocytes—relax stretches of their chromosomes, giving transcription factors easy access to inflammatory promoters and enhancers. Led by David Gate at Northwestern University in Chicago, the study tied ApoE genotype to epigenetic changes in the peripheral cells, suggesting they help explain genetic risk. In support of this, AD risk genes were among those most ramped-up.
- In AD, sections of chromatin unfold in peripheral immune cells.
- This allows for increased expression of inflammatory genes.
- ApoE4 genotype exacerbates some of these effects.
“This exciting study highlights the growing body of work supporting peripheral immune cell involvement in AD neuropathology,” wrote Mehdi Jorfi and Rudolph Tanzi of Massachusetts General Hospital in Boston in a joint comment to Alzforum. “It underscores a growing paradigm … in which the disease extends beyond solely brain-related pathology, and involves the … intricate interplay between peripheral immune cells and the brain.”
The findings dovetail with recent studies implicating systemic immune cells, particularly CD8+ T cells, in neurodegeneration. As a postdoc in Tony Wyss-Coray’s lab at Stanford University, Gate previously spotted CD8+ T cells congregating in the cerebrospinal fluid and within the brains of people with AD and PD (Jan 2020 news). Later, at Northwestern, Gate found these T cells mingling with microglia around plaques, where the two cell types expressed cognate chemokine/receptor pairs (Dec 2022 news). Similarly, in a three-dimensional cell culture model of AD, Tanzi’s group found that chemokine-recruited CD8+ T cells teamed up with microglia to destroy vulnerable neurons (Sep 2023 news).
Because epigenetic changes, such as chromatin accessibility, signify previous and ongoing responses in immune cells, first author Abhirami Ramakrishnan and colleagues decided to map them out. They used single-cell ATAC-Seq and RNA-Seq to compare both chromatin accessibility and gene expression in peripheral blood cells from 26 cognitively normal people, and 29 with AD. First, they looked for chromatin that was structurally different between the two groups. So-called differentially accessible regions (DARs) were found in most immune cell types, with CD8+ T cells having more than other cells. The majority of these regions were more accessible in people with AD than in controls, and many of the open stretches overlapped with genes involved in immune cell activation and cytokine signaling. Next, the researchers identified differentially expressed genes (DEGs) using their scRNA-Seq data. Lo and behold, a substantial proportion of these DEGs overlapped with the DARs. These overlaps—indicative of epigenetic regulation of gene expression—were most abundant in CD8+ T effector cells and monocytes. As a NeuroResource study, all this epigenetic and transcriptomic data are freely available for sifting by other researchers (see https://gatelabnu.shinyapps.io/ad_apoe_rna/).
In people with AD, what were some of the highlights of Ramakrishnan’s analysis? In monocytes, open stretches of chromatin overlapped with the gene for subunit 2 of NF-kB, and this correlated with elevated expression of the transcription factor. To Gate, this suggests these cells are in a primed, activated state. Likewise, monocytes opened chromatin near ABCA1, an AD risk gene that encodes an apolipoprotein that transfers lipids to ApoE.
CD8+ T effector memory cells bust open the CXCR3 gene promotor, and ramp up expression of this chemokine receptor at both the transcript and protein levels. Ramakrishnan spotted CXCR3+ CD8+ T cells in postmortem hippocampus and leptomeninges samples from people with AD, suggesting that this receptor might help the T cells enter the brain (image below). Indeed, Jorfi and colleagues had reported that astrocytes expressing the CXCR3 ligand, CXCL10, guide T cells to human AD neuron-glia cocultures (Jorfi et al., 2023).
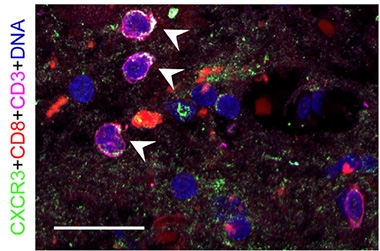
Beckoned to the Brain? T cells (CD3+, pink) expressing CD8 (red) and the chemokine receptor CXCR3 (green) loiter in the hippocampus of a person with AD (see arrows). [Courtesy of Ramakrishnan et al., Neuron, 2024.]
ApoE is the strongest AD risk gene, and the apolipoprotein it encodes has long been known to play a role in immunity and inflammation, both in and outside of the brain (for example, Gale et al., 2014; Oct 2021 news). Might ApoE genotype influence chromatin accessibility in these peripheral immune cells? To address this, the researchers stratified chromatin accessibility by APOE genotype. In a nutshell, they found a stepwise rise in the number of DARs with each copy of ApoE4. Among ApoE4 carriers, in AD, APOE3/4 and APOE4/4 monocytes had more open chromatin than APOE3/3 cells, and a corresponding uptick in transcripts encoding several inflammatory chemokines, including CCL4L2, CCL3L1, and CXCL2.
Because many AD risk genes are expressed by microglia, these brain resident immune cells have received much of the blame for driving AD risk. However, Gate pointed out that about half of risk genes are also expressed by peripheral immune cells, suggesting these cells might contribute as well. In support of this idea, the researchers identified open stretches of chromatin around several AD risk genes in immune cells from people with AD. The INPP5D gene was exposed and overexpressed in B cells and NK cells, as were clusterin and BIN1 genes in CD8+ T cells. In ApoE4 carriers, some of these associations were even stronger. “These findings suggest that AD risk factors are not contributing solely to altered microglial responses in the AD brain, but may also contribute to dysregulated peripheral immune function in AD,” the authors wrote.
To what extent AD risk genes drive disease pathogenesis via peripheral immune cells—as opposed to brain resident microglia—remains to be seen, Gate said. However, there is a possibility that the peripheral cells might lie further upstream in the AD cascade than previously thought. Gate noted that viral infections and exposure to pollutants—both of which have been linked to AD risk—can leave an epigenetic mark on peripheral immune cells. “It’s possible that these exposures contribute to AD by promoting damaging responses in these circulating cells,” he said. On the flip side, the epigenetic changes may reflect a response to the AD pathology already in the brain. Tracking these exposures and their epigenetic consequences over time will be necessary to understand how they relate to AD, he said.
To Wyss-Coray’s mind, the links between ApoE4 genotype, expression of AD risk genes, and activation of peripheral immune cells were the most intriguing in the study. “Although still controversial, a growing number of studies suggest that monocytes or related circulatory cells can enter the aging or AD brain and assume roles typically assigned to microglia,” he wrote. “If such infiltrating cells produce chemokines, they may attract other immune cells to the brain producing a possibly damaging immune environment” (comment below).
Jason Ulrich of Washington University in St. Louis wondered if APOE4 influences on peripheral immunity/inflammation could play a role in the development of cerebral amyloid angiopathy, which is linked with APOE4 in mouse models and humans. Both ApoE4 genotype and CAA have been tied to hemorrhagic side effects of amyloid-targeted immunotherapies (Aug 2023 conference news; Jan 2024 news).
Tsuneya Ikezu of the Mayo Clinic in Jacksonville, Florida, wrote that while the combination of epigenetic and transcriptomic methods in the study was a significant advance, he believes the sample size was not large enough to make comparisons by ApoE genotype. Gate acknowledged that the numbers were small, but that the cost of these extensive analyses precluded larger sample sizes, for now.—Jessica Shugart
References
News Citations
- Attack of the Clones? Memory CD8+ T Cells Stalk the AD, PD Brain
- In AD, CSF Immune Cells Hint at Mounting Mayhem in the Brain
- In 3D Cell Model of AD, Microglia and CD8+ T Cells Gang Up on Neurons
- Even In the Healthy, ApoE4 Stirs Up Trouble, Scientists Say
- Is ARIA an Inflammatory Reaction to Vascular Amyloid?
- Brain of Woman Who Died on Leqembi Shows Worst-Case Scenario
Mutations Citations
Paper Citations
- Jorfi M, Park J, Hall CK, Lin CJ, Chen M, von Maydell D, Kruskop JM, Kang B, Choi Y, Prokopenko D, Irimia D, Kim DY, Tanzi RE. Infiltrating CD8+ T cells exacerbate Alzheimer's disease pathology in a 3D human neuroimmune axis model. Nat Neurosci. 2023 Sep;26(9):1489-1504. Epub 2023 Aug 24 PubMed.
- Gale SC, Gao L, Mikacenic C, Coyle SM, Rafaels N, Murray Dudenkov T, Madenspacher JH, Draper DW, Ge W, Aloor JJ, Azzam KM, Lai L, Blackshear PJ, Calvano SE, Barnes KC, Lowry SF, Corbett S, Wurfel MM, Fessler MB. APOε4 is associated with enhanced in vivo innate immune responses in human subjects. J Allergy Clin Immunol. 2014 Jul;134(1):127-34. Epub 2014 Mar 18 PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Ramakrishnan A, Piehl N, Simonton B, Parikh M, Zhang Z, Teregulova V, van Olst L, Gate D. Epigenetic dysregulation in Alzheimer's disease peripheral immunity. Neuron. 2024 Apr 17;112(8):1235-1248.e5. Epub 2024 Feb 9 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.