Easing Microglial Brakes Alleviates Amyloid Pathology in Mice
Quick Links
Rather than giving them more gas, can microglia be driven to clear amyloid plaques by letting up on their brakes? Yes, say scientists led by Marco Colonna at Washington University in St. Louis. In the April 3 Science Translational Medicine, they describe an inhibitory microglial receptor, LILRB4, that is upregulated around plaques in Alzheimer’s and in mouse models of amyloidosis. An antibody against LILRB4 enabled microglia to curtail amyloid accumulation in mice. How? By preventing the LILRB4 ligand, ApoE, from binding the receptor, the antibody suppressed LILRB4 activation, leaving microglia free to respond to plaques.
- Microglia expressing LILRB4 surround plaques in AD cortical tissue.
- LILRB4 dampens microglial response to plaques in mice.
- Mice given a LILRB4 antibody had more phagocytic microglia, fewer plaques.
“This is an important paper given microglial activity must be carefully controlled, such as by the immune checkpoint inhibitory receptor LILRB4,” Zhiqiang An at the University of Texas Health Science Center, Houston, told Alzforum.
Others praised the research, too. “LILRB4 is a beautiful target specific to microglia engaged with plaques, and this antibody induces a novel immunomodulatory response,” said Oleg Butovsky of Brigham and Women’s Hospital in Boston. “This is a very interesting paper on microglial mechanisms in AD pathogenesis with implications for potential drug development,” Yadong Huang, University of California, San Francisco, told Alzforum.
Leukocyte Ig-like receptors (LILRs) are a group of 11 microglial markers; five activate the cells, five inhibit them. Among the latter are LILRB2 and LILRB4. LILRB2 binds toxic Aβ oligomers, leading to synapse loss (Oct 2018 news; Jun 2021 news). LILRB4 binds ApoE and is highly expressed by microglia surrounding plaques in amyloidosis mice, aka disease-associated microglia (Deng et al., 2018; Yin et al., 2023; Sep 2023 news). Genome-wide association studies pegged the locus containing both LILRB2 and LILRB4 as a risk factor for AD (Sep 2021 news; Apr 2022 news).
To understand how LILRB2 and B4 are expressed in the AD brain, co-first authors Jinchao Hou and Yun Chen ran single-nucleus RNA-Seq on microglia from postmortem cortical tissue. Compared to microglia from healthy people, those from AD cases highly expressed LILRB4 and moderately upregulated LILRB2. Immunohistochemistry revealed more LILRB4-positive microglia in AD cases, especially around amyloid plaques (image below).
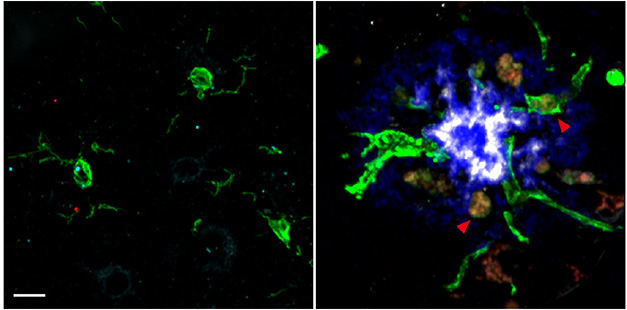
Sprouting LILRB4. While ramified microglia (green) hung out in the cortex of a healthy adult (left), microglia expressing LILRB4 (red) surrounded an ApoE-positive (white) amyloid plaque (blue) in a person who had had AD (right). [Courtesy of Hou et al., Science Translational Medicine, 2024.]
The scientists recapitulated these changes in mice by crossing 5xFAD animals with those carrying a 200-kb chunk of the human LILR gene, which encoded LILRB4 and a few other LILRs. Hou and colleagues used this construct to maintain the promotor and enhancer elements driving LILRB4 in human cells. In 6-month-old offspring, microglia expressed more LILRB4 than 5xFAD controls, and the crosses accumulated more plaques.
Intriguingly, fewer LILRB4-positive microglia surrounded amyloid plaques in the offspring, suggesting that human LILRB4 caused the cells to respond poorly to plaques. The data hint that LILRB4-positive microglia near plaques in AD may poorly clear amyloid.
Might blocking the receptor improve clearance? The scientists created an antibody against LILRB4 called ZM3.1, then injected it into the abdomens of 4-month-old 5xFAD/LILRB4 mice weekly for two months. In treated animals, 1.5 times as many Iba1-positive microglia surrounded amyloid plaques in the cortex than in controls, and the microglia upregulated phagocytic genes and engulfed almost twice as many Aβ fibrils. Treated animals also had half as many plaques in the hippocampus, cortex, and amygdala and fewer nearby dystrophic neurites (image below). Taken together, the results suggest that blocking LILRB4 switched microglia into gear to clear plaques.
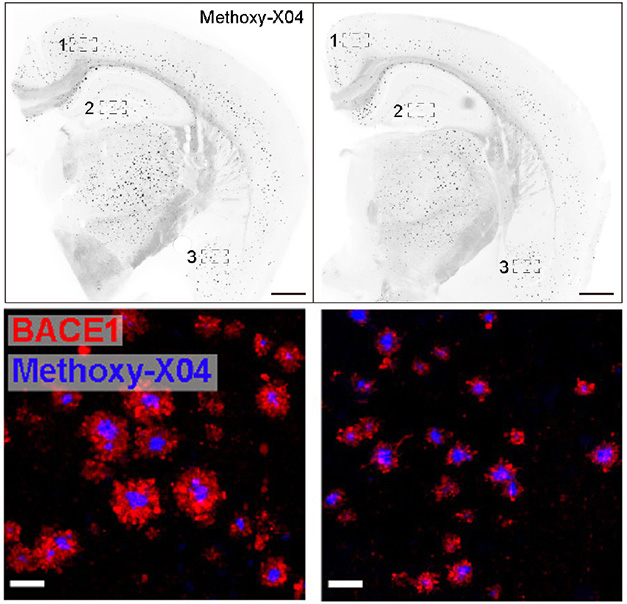
Releasing the Brakes. Compared to a 5xFAD mouse expressing human LILRB4 (left), one given a LILRB4 antibody (right) had half as many amyloid plaques (top) and half as many dystrophic neurites as measured by BACE1 levels (red, bottom). [Courtesy of Hou et al., Science Translational Medicine, 2024.]
The immunotherapy’s effects on behavior were less clear. Mice given ZM3.1 spent less time in the open arms of an elevated plus maze, i.e., had less anxiety, than untreated animals. However, they still took just as long as untreated 5xFAD mice to find an underwater platform. Nevertheless, Colonna sees this as a positive. “Changes in behavior reflect the antibody's ability to impact function,” he told Alzforum, “regardless of what that function is.”
How did ZM3.1 reduce pathology? In vitro binding experiments showed the antibody thwarting recombinant LILRB4’s binding to mouse ApoE, whether attached to Aβ fibrils or as a free lipoprotein. ZM3.1 also prevented LILRB4 from binding human ApoE3 or E4. In silico modeling and mutagenesis experiments homed in on a spot where both ApoE and ZM3.1 bind LILRB4: a loop between two extracellular Ig domains of the receptor.
All told, the authors believe that ZM3.1 binds to LILRB4 on microglia, preventing ApoE from accessing and activating the receptor. This keeps the metaphorical foot off the microglial brake, allowing the cells to respond to plaques (image below).
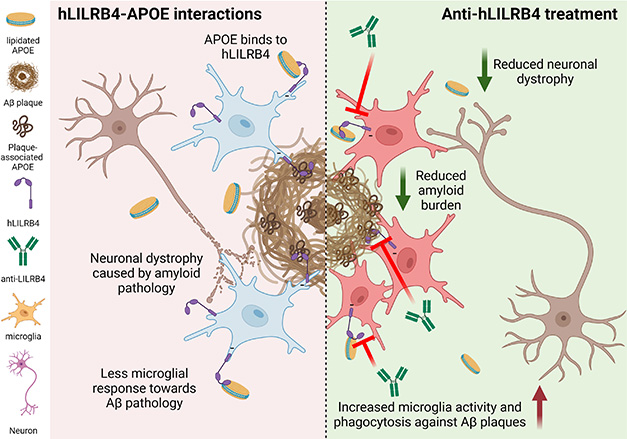
Blocking LILRB4 Unleashes Microglia. In AD (left), ApoE in lipoproteins (yellow disks) or plaques (brown squiggles) binds LILRB4 (purple hook) on microglia (blue cells). This keeps the cells silent, allowing amyloid pathology to ravage nearby neurons (brown cells). Treatment (right) with the antibody ZM3.1 (green Y) prevents ApoE-LILRB4 binding, letting microglia tame amyloid plaques and safeguard neurons. [Courtesy of Yun Chen, WashU.]
An sees therapeutic potential for ZM3.1. “It seems feasible to develop LILRB4 neutralizing antibodies to activate microglia cells to reduce plaque accumulation in AD patients,” he said. Butovsky wondered how the immunotherapy might work in APOE4 carriers, since the isoform prevents expression of disease-associated microglia genes, such as LILRB4 (Oct 2023 news).
Colonna is working with a Japanese pharmaceutical company to bring ZM3.1 into clinical trials for AD, though they do not have a timeline yet. “We still have to find the best drug to activate microglia, so it is important to test more antibodies and small molecules,” Colonna said.—Chelsea Weidman Burke
References
News Citations
- Crystal Structure of Aβ and Proposed Receptor Solved
- Aβ 'Receptors' Retard Fibrillization, Enhancing Toxicity
- PLCγ2 Variants Toggle Microglial Plaque Compactors
- From a Million Samples, GWAS Squeezes Out Seven New Alzheimer's Spots
- Paper Alert: Massive GWAS Meta-Analysis Published
- In Amyloid and Tangle Models, APOE4 Paralyzes Microglia
Research Models Citations
Paper Citations
- Deng M, Gui X, Kim J, Xie L, Chen W, Li Z, He L, Chen Y, Chen H, Luo W, Lu Z, Xie J, Churchill H, Xu Y, Zhou Z, Wu G, Yu C, John S, Hirayasu K, Nguyen N, Liu X, Huang F, Li L, Deng H, Tang H, Sadek AH, Zhang L, Huang T, Zou Y, Chen B, Zhu H, Arase H, Xia N, Jiang Y, Collins R, You MJ, Homsi J, Unni N, Lewis C, Chen GQ, Fu YX, Liao XC, An Z, Zheng J, Zhang N, Zhang CC. LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature. 2018 Oct;562(7728):605-609. Epub 2018 Oct 17 PubMed.
- Yin Z, Rosenzweig N, Kleemann KL, Zhang X, Brandão W, Margeta MA, Schroeder C, Sivanathan KN, Silveira S, Gauthier C, Mallah D, Pitts KM, Durao A, Herron S, Shorey H, Cheng Y, Barry JL, Krishnan RK, Wakelin S, Rhee J, Yung A, Aronchik M, Wang C, Jain N, Bao X, Gerrits E, Brouwer N, Deik A, Tenen DG, Ikezu T, Santander NG, McKinsey GL, Baufeld C, Sheppard D, Krasemann S, Nowarski R, Eggen BJ, Clish C, Tanzi RE, Madore C, Arnold TD, Holtzman DM, Butovsky O. APOE4 impairs the microglial response in Alzheimer's disease by inducing TGFβ-mediated checkpoints. Nat Immunol. 2023 Nov;24(11):1839-1853. Epub 2023 Sep 25 PubMed.
Further Reading
Primary Papers
- Hou J, Chen Y, Cai Z, Heo GS, Yuede CM, Wang Z, Lin K, Saadi F, Trsan T, Nguyen AT, Constantopoulos E, Larsen RA, Zhu Y, Wagner ND, McLaughlin N, Kuang XC, Barrow AD, Li D, Zhou Y, Wang S, Gilfillan S, Gross ML, Brioschi S, Liu Y, Holtzman DM, Colonna M. Antibody-mediated targeting of human microglial leukocyte Ig-like receptor B4 attenuates amyloid pathology in a mouse model. Sci Transl Med. 2024 Apr 3;16(741):eadj9052. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.