In Down's Syndrome, Blood P-Tau217 Detects Plaques and Tangles
Quick Links
In Alzheimer’s disease, a person's blood levels of Aβ42/40 and of tau correlate with his or her burden of plaques and tangles in the brain. Is the same true for Down’s syndrome? Yes, according to a cross-sectional study led by Shorena Janelidze and Oskar Hansson, Lund University, Sweden, and Benjamin Handen, University of Pittsburgh. In the July 5 JAMA Neurology, they reported that a combination of age and plasma phosphotau-217 best identified who had plaques, tangles, or faltering cognition.
- In DS, a higher plaque or tangle load meant more plasma p-tau217.
- High p-tau217 correlated with poor cognition but only in amyloid-positive participants.
- Plasma p-tau217 should simplify screening for DS clinical trials.
Just as in late-onset AD (LOAD), p-tau217 in DS seems to be a highly accurate marker of plaque pathology, with an area under the curve of 0.94. The AUC is a statistical measure of sensitivity and specificity, with 1.0 suggesting perfection. This is the first report of plasma p-tau217 in DS.
“Plasma p-tau217 is currently considered the most promising blood-based biomarker of brain tau pathology, [and this paper] demonstrates that this is also the case with DS-related AD,” Michael Rafii, University of Southern California, San Diego, wrote (full comment below). Juan Fortea, Hospital of Sant Pau, Spain, agreed. “Research on AD biomarkers in DS is catching up to that of familial and sporadic AD,” he told Alzforum.
People with DS carry an extra copy of the amyloid precursor protein gene and are destined to develop AD. To track AD pathology in DS, scientists are searching for good plasma markers. Having a blood test is especially important for people with DS, because they are less able or willing to tolerate lengthy or more-invasive procedures, such as PET scans or lumbar punctures.
In search of the best combination of plasma markers, first author Janelidze turned to the Alzheimer’s Biomarker Consortium-Down’s Syndrome (ABC-DS). She analyzed tau and amyloid PET scans, blood markers, and cognitive test scores from 300 adults with DS. Half were men, and the average age was 45.
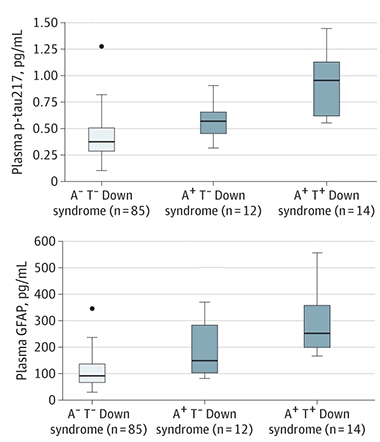
Tracking PET Status. Plasma p-tau217 (top) tracked higher in the A+T- group than in A-T-, and higher again in the A+T+ group. Plasma GFAP (bottom) occurs at much higher overall concentrations but was significantly elevated only in the A+T+ group. [Courtesy of Janelidze et al., JAMA Neurology, 2022.]
Plasma markers included total tau, p-tau217, Aβ42/Aβ40, neurofilament light (NfL), and glial fibrillary acidic protein (GFAP). The authors chose p-tau217 because it better correlated with AD pathology than did p-tau181 in LOAD, and because it is the first fluid tau marker to tick up in people who have familial AD (Jul 2020 conference news; Apr 2020 conference news; Mar 2020 news).
To learn how these markers tracked with plaques and tangles, Janelidze divided participants into those who tested PET-positive for plaques and tangles (A+T+), those who tested positive for only plaques (A+T-), and those who tested positive for neither (A-T-). Among these three groups, plasma p-tau217 was highest in A+T+ volunteers, followed by A+T-, then A-T-. GFAP was higher in the A+T+ group than either A+T- or A-T- (see image above left). T-tau was higher in both A+T+ and A+T- groups than in A-T-, while Aβ42/Aβ40 and NfL did not differ among the DS groups. All told, the authors concluded that plasma p-tau217 was the most responsive marker to changes in pathology.
When considering plaque and tangle load as continuous variables as quantified by PET, p-tau217 tracked best with both. The higher a person's PET signal, the more concentrated that marker was in his or her blood. However, this was only true in amyloid-PET-positive participants (see image below).

Tracking with Tangles, Plaques. Plasma p-tau217 correlated with neurofibrillary tangles (left) and amyloid plaques (right) in amyloid PET-positive people with Down's (blue) but not in those without plaques (orange). [Courtesy of Janelidze et al., JAMA Neurology, 2022.]
Did combining markers make the blood tests more accurate? Slightly. Plasma p-tau217 detected tau and amyloid PET positivity with AUCs of 0.94 and 0.91, respectively. Adding age, a strong driver of AD pathology in DS, bumped up the AUC to 0.96 for tangles and 0.94 for plaques. Adding any other biomarkers nudged up the AUCs by just 0.01 more.
And how did this correlate with cognition? High p-tau217 was associated with poor performance on the DS Mental State Exam and the Cued Recall Test (CRT), but again, only in amyloid-positive participants. “This shows that plasma p-tau217 tightly correlates with amyloid-induced tau phosphorylation, and it underscores how similar Alzheimer’s processes are in people with DS, familial, or sporadic AD,” Henrik Zetterberg, University of Gothenburg, Sweden, told Alzforum.
Indeed, this paper comes on the heels of two cross-sectional PET studies showing analogous tau behavior in DS and in sporadic AD. One paper reported that tau PET turns positive just after the earliest detection of plaques, confirming a lag between amyloid and tau accumulation in DS (Zammit et al., 2021). The other showed that amyloid-positive, cognitively intact people with DS who had a high tau PET load slipped faster on the CRT than those with few tangles, highlighting the close relationship between neurofibrillary tangles and cognitive decline (Hartley et al., 2022).
“[All three] papers paint the picture that, despite some differences between AD pathology in LOAD and DS, biomarker findings are similar,” wrote William Klunk at the University of Pittsburgh, who was a co-author on all the manuscripts (full comment below). Fellow co-author Beau Ances of Washington University in St. Louis agreed. “Alzheimer’s in DS follows A/T/N progression,” he said.
Scientists believe these parallels will help shape treatment and prevention trials in DS. “I think that similar biomarkers and outcome measures to those in sporadic AD trials can be used in the DS population,” said Bradley Christian, University of Wisconsin, Madison, another co-author. Rafii agreed. “The prospect of accurately staging individuals with DS within the A/T/N framework using plasma p-tau217 is a major advance in the field and sets the stage for more efficient clinical trials,” he wrote.—Chelsea Weidman Burke
References
News Citations
- Plasma p-Tau217 Set to Transform Alzheimer’s Diagnostics
- 217—The Best Phospho-Tau Marker for Alzheimer’s?
- Different CSF Phospho-Taus Match Distinct Changes in Brain Pathology
Paper Citations
- Zammit MD, Tudorascu DL, Laymon CM, Hartley SL, Ellison PA, Zaman SH, Ances BM, Johnson SC, Stone CK, Sabbagh MN, Mathis CA, Klunk WE, Cohen AD, Handen BL, Christian BT. Neurofibrillary tau depositions emerge with subthreshold cerebral beta-amyloidosis in down syndrome. Neuroimage Clin. 2021;31:102740. Epub 2021 Jun 24 PubMed.
- Hartley SL, Handen BL, Tudorascu D, Lee L, Cohen A, Piro-Gambetti B, Zammit M, Klunk W, Laymon C, Zaman S, Ances BM, Sabbagh M, Christian BT. Role of tau deposition in early cognitive decline in Down syndrome. Alzheimers Dement (Amst). 2022;14(1):e12256. Epub 2022 Apr 1 PubMed.
Further Reading
Primary Papers
- Janelidze S, Christian BT, Price J, Laymon C, Schupf N, Klunk WE, Lott I, Silverman W, Rosas HD, Zaman S, Mapstone M, Lai F, Ances BM, Handen BL, Hansson O. Detection of Brain Tau Pathology in Down Syndrome Using Plasma Biomarkers. JAMA Neurol. 2022 Aug 1;79(8):797-807. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Southern California Keck School of Mediicine
Plasma p-tau217 is currently considered the most promising blood-based biomarker of brain tau pathology. The paper by Janelidze et al. demonstrates that this is also the case with DS-related AD (DSAD) while also providing yet another example of how DSAD is similar to autosomal-dominant AD and late-onset AD. The results are part of a collaboration between the ABC-DS and Oskar Hansson’s laboratory. They show that a composite measure of p-tau217 and age showed excellent accuracy (AUC > 0.95) for distinguishing participants with DS having abnormal tau-PET scans from those with normal tau-PET scans. Moreover, increasing levels of plasma p-tau217 were found to be associated with lower cognitive performance as measured by the DS-MSE and CRT.
We first reported a correlation between cognitive decline and tau pathology as measured by PET imaging in a small cohort of adults with DS (Rafii et al., 2017). This relationship between tau pathology on PET imaging and cognitive decline has recently been confirmed using the larger cohort in ABC-DS (Hartley et al., 2022). The prospect of being able to accurately stage individuals with DS within the A/T/N framework using plasma p-tau217 is a major advance in the field and sets the stage for more efficient clinical trials.
References:
Rafii MS, Lukic AS, Andrews RD, Brewer J, Rissman RA, Strother SC, Wernick MN, Pennington C, Mobley WC, Ness S, Matthews DC, Down Syndrome Biomarker Initiative and the Alzheimer’s Disease Neuroimaging Initiative. PET Imaging of Tau Pathology and Relationship to Amyloid, Longitudinal MRI, and Cognitive Change in Down Syndrome: Results from the Down Syndrome Biomarker Initiative (DSBI). J Alzheimers Dis. 2017;60(2):439-450. PubMed.
Hartley SL, Handen BL, Tudorascu D, Lee L, Cohen A, Piro-Gambetti B, Zammit M, Klunk W, Laymon C, Zaman S, Ances BM, Sabbagh M, Christian BT. Role of tau deposition in early cognitive decline in Down syndrome. Alzheimers Dement (Amst). 2022;14(1):e12256. Epub 2022 Apr 1 PubMed.
University of Pittsburgh
The new aspect of Janelidze et al. is that plasma p-tau217 is as accurate a marker of Aβ and tau PET-detected pathology in DS as it is in late-onset AD. As in all studies, a plasma measure is much more accessible and less expensive and invasive than PET. While true in LOAD also, the even more important point in DS is that prospective trial participants or, ultimately, patients with DS, are often less willing or able to tolerate PET and MRI scans. Thus, accurate plasma biomarkers of AD are even more important in DS than in LOAD.
This paper fits together with the other three to paint the picture that, while there may be some differences between AD pathology in LOAD and DS that can be detected at autopsy (although this was a small study of 11 subjects), biomarker findings are similar in DS and LOAD. That is, Aβ can be detected very early and is related to the progression of tau deposition, which is related to cognitive decline, and plasma p-tau-217 is a good proxy for more difficult and less-accessible PET biomarkers—the latter being more important in DS than LOAD.
University of Wisconsin-Madison
Individuals with Down’s syndrome are predisposed to Alzheimer's disease, and greater than 90 percent of these individuals will develop AD pathological changes. Despite these statistics, the progression of AD in individuals with Down’s syndrome remains severely understudied, and we as researchers have a moral obligation to help this population. The Alzheimer's Biomarker Consortium-Down’s Syndrome (ABC-DS), the world’s largest Down’s syndrome research cohort aimed at studying preclinical AD progression, has set out to evaluate the early stages of AD progression through a combination of neuroimaging, neuropsychological analysis, and analysis of fluid-based AD biomarkers. The findings from the ABC-DS study will greatly help inform the recruitment of individuals with Down’s syndrome into AD clinical trials.
The study of Hartley et al. identifies that neurofibrillary tau is the driving factor of cognitive decline in individuals with Down’s syndrome based on neuroimaging and neuropsychological data. This is important because tau-based neuronal degeneration is speculated as the primary source of cognitive decline during AD in the general population. Due to the similarities, we can infer that novel AD treatments aimed at tau clearance would benefit both the general population and individuals with Down’s syndrome.
Ichimata et al. focus on case studies from postmortem individuals with Down’s syndrome. While the sample size was small, they find that AD pathology was consistent in all cases. There were some cases in which other non-AD pathologies were noted, and these pathologies would be of interest to study during the lifetime of these individuals.
My study (Zammit et al., 2021) is strictly a neuroimaging-based look at the progression of amyloid and tau in Down’s syndrome. For a time, it was speculated that there was a moderate latency period between amyloid and tau deposition during AD progression. However, through advancement in PET image quantification techniques, we found that tau begins to spread during the early stages of amyloid progression. Because of this, individuals with Down’s syndrome would greatly benefit from early intervention treatments targeting both amyloid and tau in the hope of hindering or preventing the onset of AD dementia.
The most recent study of Janelidze et al. is the first to evaluate the novel blood-based biomarker, plasma p-tau217, in the Down’s syndrome population. Plasma p-tau217 is a very accurate and sensitive measure of neurofibrillary tau, and the collection of p-tau217 is much less invasive compared to obtaining CSF measures of tau. P-tau217 is speculated as a very early indicator of AD progression and can simplify the AD screening process for clinical trials. Because the Down’s syndrome population would greatly benefit from early intervention studies, as noted in the above studies, collection of p-tau217 would greatly facilitate their recruitment.
References:
Hartley SL, Handen BL, Tudorascu D, Lee L, Cohen A, Piro-Gambetti B, Zammit M, Klunk W, Laymon C, Zaman S, Ances BM, Sabbagh M, Christian BT. Role of tau deposition in early cognitive decline in Down syndrome. Alzheimers Dement (Amst). 2022;14(1):e12256. Epub 2022 Apr 1 PubMed.
Ichimata S, Yoshida K, Visanji NP, Lang AE, Nishida N, Kovacs GG. Patterns of Mixed Pathologies in Down Syndrome. J Alzheimers Dis. 2022;87(2):595-607. PubMed.
Zammit MD, Tudorascu DL, Laymon CM, Hartley SL, Ellison PA, Zaman SH, Ances BM, Johnson SC, Stone CK, Sabbagh MN, Mathis CA, Klunk WE, Cohen AD, Handen BL, Christian BT. Neurofibrillary tau depositions emerge with subthreshold cerebral beta-amyloidosis in down syndrome. Neuroimage Clin. 2021;31:102740. Epub 2021 Jun 24 PubMed.
University of Cambridge
The characterization of blood-based biomarkers not only is important for clinical practice and in preventive clinical trials as outcome measures, but they also shed light on possible underlying mechanisms of disease. The paper by Janelidze et al. describes a cross-sectional study in a reasonably sized cohort of people with Down’s syndrome (DS). It identifies p-tau217 (allowing for age) as a promising plasma biomarker of brain tauopathy as measured by PET, and shows a good correspondence to amyloid (A+/-) and tau (T+/-) staging.
The study shows that some other popular candidate biomarkers including NfL appear to be less sensitive. Biomarkers can obviate the need for PET, keeping costs low.
The paper also shows that GFAP, which is a marker of astroglial injury and which has been shown to associate well with the degree of brain injury post-trauma, is another correlate of pathology in the DS cohort. Astrocytes are likely to be playing an important role in disease progression, not just because of the overexpression of S100B (located on the triplicated chromosome-21 of DS), but also because of the likely immune activation.
Future longitudinal studies will indicate the degree of closeness with specific pathology. The study also provides further evidence of the heterogeneity amongst the “genetic forms of AD” (familial autosomal-dominant AD, and the DS group) and late-onset or sporadic AD. Although in the late-onset forms of AD age-dependent confounds and co-occurring pathologies occur more frequently, neuropathology case studies of DS increasingly recognize some degrees of variations of the regional distribution of tau or amyloid and pathologies such as Lewy bodies and TDP-43 (Ichimata et al., 2022; Davidson et al., 2018).
References:
Ichimata S, Yoshida K, Visanji NP, Lang AE, Nishida N, Kovacs GG. Patterns of Mixed Pathologies in Down Syndrome. J Alzheimers Dis. 2022;87(2):595-607. PubMed.
Davidson YS, Robinson A, Prasher VP, Mann DM. The age of onset and evolution of Braak tangle stage and Thal amyloid pathology of Alzheimer's disease in individuals with Down syndrome. Acta Neuropathol Commun. 2018 Jul 4;6(1):56. PubMed.
Make a Comment
To make a comment you must login or register.