Does a Rare Fibronectin Variant Protect Against APOE4?
Quick Links
APOE4 strongly increases the risk of sporadic Alzheimer’s disease. Yet some carriers evade the disease entirely. Now, scientists led by Richard Mayeux, Caghan Kizil, and Badri Vardarajan of Columbia University in New York peg some of that protection to a missense mutation in the gene for fibronectin. In the April 10 Acta Neuropathologica, they reported that APOE4 carriers with a glycine-to-glutamic-acid mutation in fibronectin were 71 percent less likely to get AD. If they did get the disease, it was 3.5 years later than expected. In a neuropathology cohort, APOE4 carriers who had been cognitively healthy had less fibronectin surrounding blood vessels in the brain than did those who had died having AD. Though these resilient cases did not carry the protective fibronectin mutation, the data supports the authors’ contention that the variant limits pathological accumulation of the extracellular matrix protein.
- Some APOE4 carriers are resilient to Alzheimer’s.
- A glycine-to-glutamic-acid substitution at amino acid 357 of fibronectin reduces their risk of AD.
- Resilient E4 carriers had limited fibronectin surrounding blood vessels and minimal gliosis in the brain.
Joel Blanchard and Braxton Schuldt at the Icahn School of Medicine at Mount Sinai, New York, called the work exciting. “While previous literature strongly supports a link between the extracellular matrix and AD, Bhattarai and colleagues uniquely introduce APOE4 to this discussion and highlight potential protective variants,” they wrote (comment below).
One copy of APOE4 increases AD risk two- to threefold, while having two alleles ups risk 10 to 15 times. Still, some E4 carriers stay sharp into their 70s and 80s. In search of protective gene variants that might explain this resiliency, co-first authors Prabesh Bhattarai and Tamil Iniyan Gunasekaran at Columbia analyzed whole-genome sequencing data on 2,430 people 60 years or older from three cohorts: the National Institute on Aging Alzheimer's Disease Family Based Study (NIA-AD FBS), the Washington Heights/Inwood Columbia Aging Project (WHICAP), and the Estudio Familiar de Influencia Genetica en Alzheimer (EFIGA) cohort. Of these, 379 people had two copies of APOE4 and 1,162 people had one. Half of the participants had AD.
The authors searched for rare coding variants, i.e., frequencies below 1 percent. They identified 510 mutations in 476 genes among cognitively healthy APOE4/4 carriers that were not found in the E4 carriers with AD, or in the E4 noncarriers, hinting that the variants protect. Of these, 56 lay in proteins found in the extracellular matrix (ECM), especially the basement membrane of the blood-brain barrier. The ECM surrounding BBB vessels thickens during AD (Lepelletier et al., 2015; reviewed by Sun et al., 2021).
Bhattarai and colleagues focused on the rs140926439 missense coding variant in the fibronectin gene, FN1, because it stuck out as a top hit in all three cohorts. The variant also caught the attention of co-first author Michael Belloy, at the Washington University School of Medicine in St. Louis, because he had independently found that the mutation was protective in three additional cohorts: the Alzheimer’s Disease Genetic Consortium (ADGC), the Alzheimer’s Disease Sequencing Project (ADSP), and the UK Biobank. Among 7,185 people 60 or older in those cohorts who carried two copies of APOE4, rs140926439 carriers had 71 percent less chance of getting dementia. In rs140926439 carriers who did have AD, they developed symptoms 3.5 years later than noncarriers, on average. The researchers decided to combine their results.
How does the variant protect? Three pathogenicity algorithms, SIFT, REVEL, and MetaL R, predicted the glycine-to-glutamic-acid mutation would alter the structure of the protein sufficiently to affect function. To test this idea, the scientists turned to zebrafish because they had extensively characterized the effect of amyloidosis on the BBB in these animals and because the barrier is highly conserved among vertebrates. After injecting Aβ42 into the cerebral ventricle, amyloid accumulates in neurons throughout the zebrafish brain, microglia become activated, synapses degenerate, and astroglia detach from blood vessels (Bhattarai et al., 2016; Lee et al., 2022). Brain vascular smooth muscle also churns out 20 percent more fibronectin.
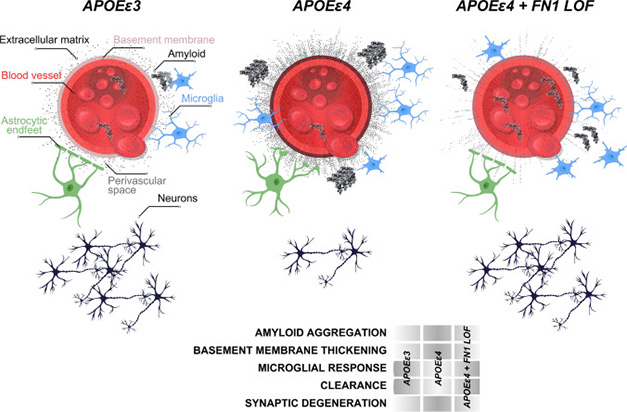
Thin Fibronectin Protects. Around blood vessels in APOE3 carriers (left), microglia (blue) clear amyloid plaques (black chevrons) with the help of astrocytes (green) that communicate with the vessels. In APOE4 carriers (middle), the extracellular matrix (gray) surrounding blood vessels thickens, limiting plaque clearance and hindering astrocyte-blood vessel connections. In E4 carriers with the fibronectin variant (right), the extracellular matrix remains thin, allowing amyloid clearance and astrocyte contacts. [Courtesy of Bhattarai et al., Acta Neuropathologica, 2024.]
When the scientists knocked out FN1B, the zebrafish ortholog, then injected Aβ42, the peptide roused 25 percent fewer astroglia, especially around the brain vasculature, and 60 percent more phagocytic microglia were retained. The zebrafish also had as many synapses as wild-types. To the authors, this suggested that fibronectin loss-of-function improves glial responses to amyloid to protect neurons, possibly by thinning the ECM and allowing the cells to clear more amyloid (image above).
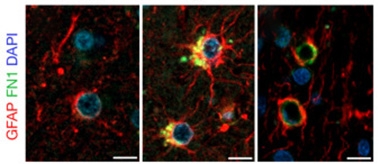
Protected Vessels? Astrocytes (red) and fibronectin (green) surround blood vessels in the brains of APOE3/3 (left) and E4/4 (right) carriers who are cognitively healthy. A thicker ECM and a greater number of astrocytes surround vessels in APOE4/4 carriers who had had AD (middle). [Courtesy of Bhattarai et al., Acta Neuropathologica, 2024.]
There is some evidence that fibronectin might hamper amyloid clearance in people, too. In prefrontal cortex tissue from eight APOE4/4 carriers who had had AD, immunostaining showed 27 percent more fibronectin surrounding BBB basement membrane than in 11 APOE3/3 AD cases. Further, in six APOE4 homozygotes who did not have AD, vessels looked like those from cognitively normal E3s. Together, the findings hint that maintaining a thin ECM around BBB vessels protects against AD (image at right). Notably, none of the brain tissue donors carried the rare FN1 rs140926439 variant.
The findings tie in with prior work that fibronectin thickening around BBB vessels exacerbates cerebral amyloid angiopathy and vascular damage (Wyss-Coray et al., 2000). “Together, these [studies] suggest that vascular fibronectin accumulation and basement membrane thickening may promote pathological vascular Aβ, and that mutations preventing this could be protective,” wrote Andrew Yang of the University of California, San Francisco.—Chelsea Weidman Burke
References
Mutations Citations
Paper Citations
- Lepelletier FX, Mann DM, Robinson AC, Pinteaux E, Boutin H. Early changes in extracellular matrix in Alzheimer's disease. Neuropathol Appl Neurobiol. 2015 Nov 6; PubMed.
- Sun Y, Xu S, Jiang M, Liu X, Yang L, Bai Z, Yang Q. Role of the Extracellular Matrix in Alzheimer's Disease. Front Aging Neurosci. 2021;13:707466. Epub 2021 Aug 27 PubMed.
- Bhattarai P, Thomas AK, Cosacak MI, Papadimitriou C, Mashkaryan V, Froc C, Reinhardt S, Kurth T, Dahl A, Zhang Y, Kizil C. IL4/STAT6 Signaling Activates Neural Stem Cell Proliferation and Neurogenesis upon Amyloid-β42 Aggregation in Adult Zebrafish Brain. Cell Rep. 2016 Oct 18;17(4):941-948. PubMed.
- Lee AJ, Raghavan NS, Bhattarai P, Siddiqui T, Sariya S, Reyes-Dumeyer D, Flowers XE, Cardoso SA, De Jager PL, Bennett DA, Schneider JA, Menon V, Wang Y, Lantigua RA, Medrano M, Rivera D, Jiménez-Velázquez IZ, Kukull WA, Brickman AM, Manly JJ, Tosto G, Kizil C, Vardarajan BN, Mayeux R. FMNL2 regulates gliovascular interactions and is associated with vascular risk factors and cerebrovascular pathology in Alzheimer's disease. Acta Neuropathol. 2022 Jul;144(1):59-79. Epub 2022 May 24 PubMed.
- Wyss-Coray T, Lin C, Sanan DA, Mucke L, Masliah E. Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer's disease-like microvascular degeneration in transgenic mice. Am J Pathol. 2000 Jan;156(1):139-50. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Bhattarai P, Gunasekaran TI, Belloy ME, Reyes-Dumeyer D, Jülich D, Tayran H, Yilmaz E, Flaherty D, Turgutalp B, Sukumar G, Alba C, McGrath EM, Hupalo DN, Bacikova D, Le Guen Y, Lantigua R, Medrano M, Rivera D, Recio P, Nuriel T, Ertekin-Taner N, Teich AF, Dickson DW, Holley S, Greicius M, Dalgard CL, Zody M, Mayeux R, Kizil C, Vardarajan BN. Rare genetic variation in fibronectin 1 (FN1) protects against APOEε4 in Alzheimer's disease. Acta Neuropathol. 2024 Apr 10;147(1):70. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.