Deep Brain Stimulation: It’s All About the Rhythm
Quick Links
For decades, deep brain stimulation has been an effective treatment for the motor symptoms of Parkinson’s disease (PD). However, researchers have been unsure how an electrode buried deep in the brain imparts sometimes dramatic effects. In the April 13 Nature Neuroscience, researchers led by Philip Starr, University of California, San Francisco, propose a new idea. They claim that DBS restores the normal coupling of two brain network rhythms, β and γ, which becomes too strong in PD. By uncoupling them, DBS frees up brain circuits needed for movement. “This lab presents the first evidence that this phase-amplitude coupling could be pathologically abnormal,” said Robert Knight, University of California, Berkeley, who was not involved in the research. “If you simply disrupt the coupling by a slight jolt, it returns to a normal level, and the patient moves fluidly.”
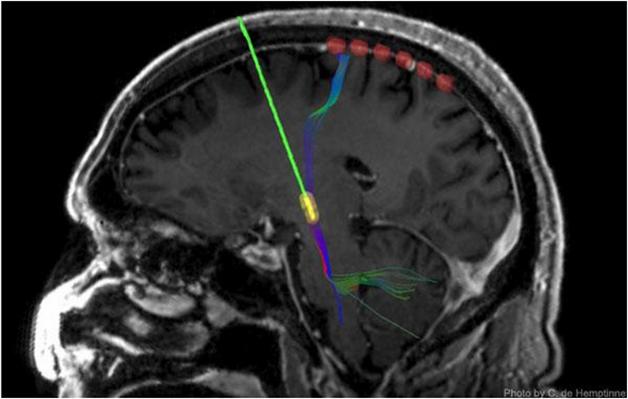
Keeping Tabs on DBS: A strip of six recording electrodes (red) measure output from the motor cortex after surgeons implant a DBS device (green/yellow). [Image courtesy of C. de Hemptinne.]
Deep brain stimulation has helped many Parkinson’s patients whose symptoms persisted despite medication. A surgeon implants an electrode into the thalamus, subthalamic nucleus, or globus pallidus. It connects via a wire to a pacemaker under the skin of the chest, and the pacemaker then sends electrical signals to the electrode. This stimulates nearby neurons, often reducing motor symptoms and the need for medication.
The technology is far from perfect, and some patients do not respond. Researchers have a hard time improving it when they are not sure exactly how it works. Several studies suggest the therapy reduces excessively synchronized β oscillations in deep brain regions (Eusebio et al., 2011). These oscillations—which fluctuate at about 20 Hz—make up one of the cardinal rhythms of the motor system. Whether the DBS effect extends to β rhythms in the motor cortex is controversial. Some studies report reduced cortical β power in PD patients who receive successful DBS or pharmaceutical treatment, while others find it increased (Whitmer et al., 2012; Melgari et al., 2014). Starr’s group wanted to better understand the effect on the motor cortex, which ultimately generates movements.
Two years ago, first author Coralie de Hemptinne and colleagues reported that in the cortices of patients with PD, phase-amplitude coupling (PAC) was particularly strong (de Hemptinne et al., 2013). PAC is one way neurons communicate with each other. The researchers measured PAC by using temporary cortical strip electrodes that lie at the brain’s surface and record electrical signals (see image above). In this sort of neural network coupling, an oscillation with a slower rhythm dictates the amplitude of a faster one. In this case, at the troughs of slow β oscillations (13-30 Hz), fast γ oscillations (50-200 Hz) reach their peak amplitude. In a healthy brain, this PAC is flexible and dynamic. It is moderately strong during rest, and disappears during the preparation and execution of movement, freeing up neurons to engage in a task. However, de Hemptinne and colleagues found that PAC is excessive in the neurons of the motor cortex of PD patients at rest and stays strong during movement. In two patients, the researchers saw that DBS reduced PAC, bringing it back to normal. Might this be the mechanism by which DBS improves movement in PD?
To find out, the researchers examined 23 more PD patients who were having DBS electrodes implanted in their subthalamic nucleus (STN). During surgery, the scientists temporarily inserted a flat, flexible strip of electrodes just underneath the skull and on top of the motor cortex to measure the activity of nearby neurons while DBS was switched on and off.
The researchers then calculated the PAC between β and γ oscillations while the patient either rested or performed a reaching task. They tested the volunteers before, during, and after STN stimulation.
During rest, DBS led to a considerable drop in cortical coupling. This rebounded soon after stimulation stopped. At the same time, DBS reduced the patient’s muscle rigidity scores on the Unified Parkinson’s Disease Rating Scale. A strong PAC associated with more rigidity before and after DBS. However, the reductions in PAC did not correlate with less rigidity, possibly because the latter varied only a small amount, the authors wrote.
As patients reached to touch a blue dot on a screen in front of them, PAC responded similarly to DBS. Before the deep brain electrode was turned on, PAC fell somewhat as the person prepared to reach out and when he or she reached, though the movements were still slow. With DBS turned on, however, PAC fell even more and patients reached out more quickly. The results suggest that DBS works by reducing excess PAC. The authors propose that electrode stimulation increases noise in the system, disrupting oscillations that are too tightly coupled, and allowing neurons to engage in specific tasks, de Hemptinne said.
De Hemptinne said that she cannot be sure that tighter coupling causes the symptoms of Parkinson’s. She believes PAC could be a biomarker researchers use to improve DBS. For instance, an electrode strip that continuously detects coupling activity at the cortex and automatically adjusts DBS to bring PAC back to normal might improve the outcome. The researchers are currently testing a prototype in five patients. Each has an experimental implant. For now, the researchers are observing PAC changes over time, figuring out how DBS and medication affect it, and how that correlates with symptoms. They have not yet tried to modulate the deep brain stimulation based on that data.
“If we could better measure the electrical activity of many brain cells, we might build more intelligent neurostimulators that keep an eye out for pathological communication and stimulate as needed,” agreed Bradley Voytek, University of California, San Diego. As DBS has proven successful in a wide range of other disorders, such as depression and obsessive-compulsive disorder, these results could have far-reaching implications, he wrote to Alzforum. He pointed out that while the study is large for its kind, the sample size is still small.
Michael Okun, University of Florida, Gainesville, said scientists should be cautious about over-interpretation because the changes in β synchrony may not be a biomarker for all PD symptoms. “Various PD symptoms may be associated with different neural oscillations. We will likely need to tailor future therapies to the oscillation, and to the disabling symptom.”
Beyond PD therapy, this study could have implications for a broader understanding of brain physiology, Knight said. “For a long time, oscillatory networks were viewed as epiphenomena of brain activity,” he told Alzforum. However, emerging evidence suggests that these oscillations are an active part of communicating, remembering, and thinking. “Now, Starr's lab has shown it to be abnormal and malleable in a major neurological disorder. Hopefully this will make more people open to the importance of oscillatory networks.”—Gwyneth Dickey Zakaib
References
Paper Citations
- Eusebio A, Thevathasan W, Doyle Gaynor L, Pogosyan A, Bye E, Foltynie T, Zrinzo L, Ashkan K, Aziz T, Brown P. Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatry. 2011 May;82(5):569-73. Epub 2010 Oct 9 PubMed.
- Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson's disease. Front Hum Neurosci. 2012;6:155. Epub 2012 Jun 4 PubMed.
- Melgari JM, Curcio G, Mastrolilli F, Salomone G, Trotta L, Tombini M, di Biase L, Scrascia F, Fini R, Fabrizio E, Rossini PM, Vernieri F. Alpha and beta EEG power reflects L-dopa acute administration in parkinsonian patients. Front Aging Neurosci. 2014;6:302. Epub 2014 Nov 5 PubMed.
- de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, Ostrem JL, Galifianakis NB, Starr PA. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A. 2013 Mar 19;110(12):4780-5. Epub 2013 Mar 7 PubMed.
Further Reading
Papers
- Cagnan H, Duff EP, Brown P. The relative phases of basal ganglia activities dynamically shape effective connectivity in Parkinson's disease. Brain. 2015 Apr 16; PubMed.
- Beudel M, Little S, Pogosyan A, Ashkan K, Foltynie T, Limousin P, Zrinzo L, Hariz M, Bogdanovic M, Cheeran B, Green AL, Aziz T, Thevathasan W, Brown P. Tremor Reduction by Deep Brain Stimulation Is Associated With Gamma Power Suppression in Parkinson's Disease. Neuromodulation. 2015 Apr 16; PubMed.
- Yanagisawa T, Yamashita O, Hirata M, Kishima H, Saitoh Y, Goto T, Yoshimine T, Kamitani Y. Regulation of motor representation by phase-amplitude coupling in the sensorimotor cortex. J Neurosci. 2012 Oct 31;32(44):15467-75. PubMed.
- Goutagny R, Gu N, Cavanagh C, Jackson J, Chabot JG, Quirion R, Krantic S, Williams S. Alterations in hippocampal network oscillations and theta-gamma coupling arise before Aβ overproduction in a mouse model of Alzheimer's disease. Eur J Neurosci. 2013 Jun;37(12):1896-902. PubMed.
News
- X Marks the Spot? Nucleus Basalis for DBS in Alzheimer’s
- Brain Stimulation Turns Paralyzed Rats into Walkers, Swimmers
- Deep-Brain Stimulation: Decade of Surgical Relief, Not Just for PD
- Brain Electrodes Help Early PD, But Still Risky and Costly
- Zapping the Brain Can Help, But Where, and With What?
- Deep-Brain Stimulation Improves Connectivity in AD
Primary Papers
- de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, Starr PA. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson's disease. Nat Neurosci. 2015 Apr 13; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.