CSF Hugs Arteries to Squeeze into the Brain
Quick Links
The space between the arachnoid and pia meningeal layers encasing the brain is a landscape of connective tissue, blood vessels, and cerebrospinal fluid. Scientists debate how that fluid moves within the space and through brain tissue. Now, an MRI study by Per Kristian Eide and Geir Ringstad of the Oslo University Hospital in Norway supports the idea that people have a glymphatic system. In the March 5 Nature Communications, they reported serial scans showing CSF flowing into the brain by hugging cerebral arteries in the subarachnoid space. Within minutes, the fluid seeped into adjacent brain tissue. This flow was slowed in people with hydrocephalus, a disorder that causes a buildup of fluid in the brain.
- In people, CSF flows into the brain around arteries.
- Within minutes, it percolates into the parenchyma.
- Hydrocephalus slows this movement.
“This paper shows that the subarachnoid space is divided into different compartments—the perivascular space and the outer subarachnoid space,” said Maiken Nedergaard of the University of Rochester Medical Center in New York. “This is important for glymphatic clearance, enabling clean CSF to flush in, and dirty fluid to exit, separately.”
The glymphatic clearance, a lymph-like process that relies on astrocytes to wash CSF through the brain, has been documented in mice. The fluid flows into brain tissue through perivascular spaces along arteries and drains out along veins (Aug 2012 news). Supporting this, a recent mouse imaging study showed CSF exiting the brain along small bridging veins. It slipped out the arachnoid membrane through openings called “cuffs” around these vessels into the dura mater and on into lymph vessels (Feb 2024 news).
Previously, Eide and Ringstad had focused on CSF movement within the brain, tracking an MRI tracer as it flowed next to arteries without penetrating them (Oct 2022 news). To map how CSF entered the brain, Eide analyzed consecutive MRI scans from 75 adults intrathecally injected with the tracer gadobutrol. Of these participants, 14 were healthy, 22 had idiopathic normal-pressure hydrocephalus (iNPH), and the rest had other CSF disorders, such as arachnoid or pineal cysts, or intracranial hypo- or hypertension. Participants were 50, on average, and two-thirds were women.

CSF Doughnut. In the subarachnoid space (SAS) of the cerebral cortex (CC), gadobutrol (white, top left; blue, top right) gathers in the perivascular subarachnoid space (PVSAS) around an artery (A). Three-dimensional models (bottom) of the MRI scans depict the pia (P), perivascular membrane (PVM), and the arachnoid trabeculae (AT)—strands of connective tissue connecting the arachnoid and pial membranes. [Courtesy of Eide et al., Nature Communications, 2024.]
Over an average of 14 minutes, the tracer traveled from the injection site in the lumbar spine to the basal cisterns, i.e., CSF reservoirs, at the base of the skull. Then, it concentrated around the anterior, middle, and posterior cerebral arteries, forming perivascular rings (image at right).
After the tracer surrounded arteries, it followed them into the parenchyma. Within minutes, the tracer diffused into the cerebrum into which the arteries feed, permeating the frontal cortex, and temporal cortex. Two hours after injection, the tracer had seeped deep within the brain (image below). That the tracer ended up in cortical tissue suggested to Eide that this CSF inflow feeds into the glymphatic system.
Because there was a delay before the tracer percolated from the perivascular space into the parenchyma, the authors believe that a semi-permeable membrane encircles the subarachnoid space around arteries to create CSF channels. However, the low resolution of MRI prevented them from seeing this membrane. Researchers led by the late Roy Weller had proposed such a membrane might regulate retrograde flow of CSF from the brain along arteries, but Eide and Ringstad believe that the flow must be antegrade (Zhang et al., 1990).
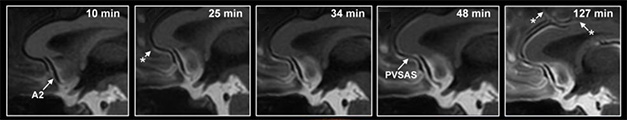
Creeping Up. An MRI tracer enters the basal cisterns (left panel, white area at bottom), then flows through perivascular subarachnoid space (PVSAS) next to the anterior cerebral artery (A2) into the brain, seeping into the parenchyma. Asterisks indicate the tracer’s appearance at different spots along the artery. [Courtesy of Eide et al., Nature Communications, 2024.]
Might CSF flow change in people with brain disorders? Indeed, the fluid trekked sluggishly in people with normal-pressure hydrocephalus, despite their having enlarged perivascular subarachnoid spaces. The tracer took 15 minutes longer to appear at the anterior cerebral artery than it did in controls, and 2.5-fold less trickled into the cortex two hours after injection. The authors chalk this up to high intracranial pulse pressure from enlarged ventricles. They think this restricts artery pulses, which slow perivascular CSF movement, as the pulses gently push the fluid along.
Notably, the scientists rarely saw the tracer around veins. Though veins are slightly harder to see on MRI than arteries, Eide does not think this is simply a methodological issue. “If there was perivenous tracer enrichment, I expect we would see it,” he told Alzforum. This made sense to Nedergaard, who also sees no CSF outflow in mouse cortical veins, instead spotting it in deep, central veins near the neck. Because veins do not pulsate as strongly as arteries, she thinks the cortical perivenous spaces might not have enough pressure to push CSF out.—Chelsea Weidman Burke
References
News Citations
- Brain Drain—“Glymphatic” Pathway Clears Aβ, Requires Water Channel
- Meningeal Cuffs Around Veins Form Exit and Entry Ramps to the Brain
- In Hydrocephalus, Slow Drainage May Cause Dementia
Paper Citations
- Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat. 1990 Jun;170:111-23. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Eide PK, Ringstad G. Functional analysis of the human perivascular subarachnoid space. Nat Commun. 2024 Mar 5;15(1):2001. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.