Courtesy of CryoEM: GABAA Receptor in Close-Up View
Quick Links
Using cryo-electron microscopy, scientists have gotten their first atomic snapshot of a human GABAA receptor. This inhibitory channel is the target of a range of anesthetic, anxiolytic, and recreational drugs. It is also the target for allopregnanolone, a neurosteroid metabolite in clinical trials for Alzheimer’s disease. Dysfunction at this receptor plays a role in disorders such as autism, insomnia, and epilepsy. Exposing its nooks and crannies in the June 27 Nature, scientists reveal the exact spots where the GABA neurotransmitter touches the receptor. They also capture the receptor bound to an antagonist for benzodiazepines. Understanding the receptor’s architecture in detail will help scientists design better compounds that modify the channel, said senior author Ryan Hibbs, University of Texas Southwestern Medical Center, Dallas.
- CryoEM reveals three-dimensional atomic structure of the GABAA receptor.
- Binding pockets for GABA and flumazenil wedge between subunits.
- Results could allow better design of drugs to target the receptor.
“Now we have the first reliable structural information for one of biggest drug targets in the human brain,” he told Alzforum. Understanding how diverse compounds work through this receptor will help scientists design new drugs with fewer off-target effects, he said.
“This work is an outstanding collection of data on the synaptic isoform of the human GABAA receptor,” Graziano Pinna, University of Illinois, Chicago, wrote to Alzforum (full comment below). “It will help us understand why current drugs fail to exert pharmacological properties by acting at GABAA receptors and help anticipate future design of agents for the treatment of neurological and psychiatric disorders.”

From The Top.
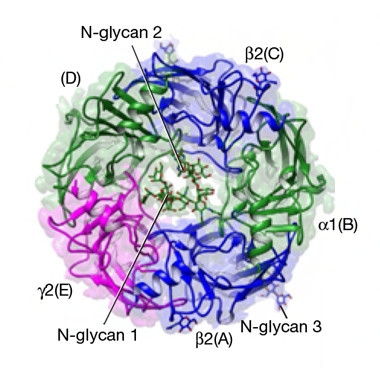
Bird’s-eye view of this GABAA receptor shows its α1, β2, and γ2 subunits gathered around a central ion channel. [Courtesy of Zhu et al., 2018, Nature.]
In GABAA receptors, five subunits band together to form a channel that allows chloride ions to flow into the neuron. These five subunits come from a pool of 19 possible candidates. Researchers previously used cryoEM to zoom in on a GABAA receptor made of five β3 subunits, but this isoform is not found physiologically (Miller and Aricescu, 2014). To image an isoform involved in human disease, first author Shaotong Zhu and colleagues purified and froze the most common GABAA receptor in the human brain—comprising two α1-, two β2-, and one γ2-subunit—and imaged it with an electron microscope.
Achieving an average resolution of 3.9 angstroms, the scientists viewed the receptor bound to two ligands: the GABA neurotransmitter or flumazenil, a drug that blocks benzodiazepines, decades-old drugs that allosterically enhance GABA’s effect on the receptor. Benzodiazepines act as sedatives, anesthetics, anticonvulsants, calming agents in panic attacks, and muscle relaxants; flumazenil counteracts them.

Side View.
GABAA receptor subunits gather in a cylinder, stabilized here by antibody fragments (Fab). Outer and inner membrane boundaries are marked. [Courtesy of Zhu et al., 2018. Nature.]
Zhu and colleagues show that the subunits of this GABAA receptor join up in a cylinder, where β2-α1-β2-α1-γ2 form an inner channel (see image above). Each subunit is tall and thin, with a single extracellular α-helix that sits atop 10 folded β-strands. Four more α-helices curl up underneath, one of which lines the ion channel.
GABA binds the receptor in the extracellular region at the interfaces between both β2 and α1-subunits (see image at right). There, the neurotransmitter is enveloped by amino acids whose side chains complement its negative and positive ends.
While flumazenil also binds extracellularly, it occupies the crevice between α1 and γ2 subunits (see image below). Here again, this pocket is lined by specific amino acids that complement the molecular structure of flumazenil to form aromatic, electrostatic, and hydrogen bonds.
The Spaces in Between.
In this GABAA receptor, GABA (red) binds between β2-α1 and flumazenil (cyan) binds between α1- γ2. Black and yellow boxes indicate regions that could be targeted by new allosteric modulators. [Courtesy of Zhu et al., 2018. Nature.]
However, neither compound occupies the interface formed between the α1 and β2-subunits, or that between γ2 and β2. These two areas are potential gold mines for chemists who want to design new drugs that target GABAA allosterically to enhance GABA function, Hibbs said.
The authors were surprised to see that the two α subunits had bulky sugars that protruded into the channel’s pore. This likely explains why GABAA receptors contain at most two α subunits; the pore cannot accommodate more.
Hibbs said the mechanism for opening the GABAA receptor remains a hotly debated question. In future work, he wants to image the receptor before, during, and after it opens to get a better sense of how it allows chloride ions to pass through.
Is this set of structures useful for Alzheimer’s? Possibly, said Hibbs. Seizures are commonly observed in AD patients. Agents that potentiate GABAA receptors can calm this hyperexcitation in the brain, which could be useful for some patients, he said. The GABAA receptor occasionally rears its head in AD research. For example, inhibition spikes in hippocampal neurons of APP transgenic mice in response to hyperactivity (e.g., Palop et al., 2007).
On the clinical front, researchers still debate whether using benzodiazepines long-term increases a person’s risk for dementia (e.g., Zhong et al., 2015; Gray et al., 2016; Picton et al., 2018; Calvo-Flores Guzmán et al., 2018).
Specifically, understanding the structure of GABAA receptors could help determine the consequences of isoform substitutions that happen over the course of disease, Hibbs said (Kwakowsky et al., 2018).
In future work, Hibbs said he plans to explore how other classes of drugs, including anesthetics, interact with the GABAA receptor.—Gwyneth Dickey Zakaib
References
Therapeutics Citations
Paper Citations
- Miller PS, Aricescu AR. Crystal structure of a human GABAA receptor. Nature. 2014 Aug 21;512(7514):270-5. Epub 2014 Jun 8 PubMed.
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007 Sep 6;55(5):697-711. PubMed.
- Zhong G, Wang Y, Zhang Y, Zhao Y. Association between Benzodiazepine Use and Dementia: A Meta-Analysis. PLoS One. 2015;10(5):e0127836. Epub 2015 May 27 PubMed.
- Gray SL, Dublin S, Yu O, Walker R, Anderson M, Hubbard RA, Crane PK, Larson EB. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ. 2016 Feb 2;352:i90. PubMed.
- Picton JD, Marino AB, Nealy KL. Benzodiazepine use and cognitive decline in the elderly. Am J Health Syst Pharm. 2018 Jan 1;75(1):e6-e12. PubMed.
- Calvo-Flores Guzmán B, Vinnakota C, Govindpani K, Waldvogel HJ, Faull RL, Kwakowsky A. The GABAergic system as a therapeutic target for Alzheimer's disease. J Neurochem. 2018 Sep;146(6):649-669. Epub 2018 Aug 1 PubMed.
- Kwakowsky A, Calvo-Flores Guzmán B, Pandya M, Turner C, Waldvogel HJ, Faull RL. GABAA receptor subunit expression changes in the human Alzheimer's disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. J Neurochem. 2018 Jun;145(5):374-392. PubMed.
Further Reading
Papers
- Rissman RA, Mobley WC. Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer's disease. J Neurochem. 2011 May;117(4):613-22. PubMed.
- Luchetti S, Huitinga I, Swaab DF. Neurosteroid and GABA-A receptor alterations in Alzheimer's disease, Parkinson's disease and multiple sclerosis. Neuroscience. 2011 Sep 15;191:6-21. PubMed.
Primary Papers
- Zhu S, Noviello CM, Teng J, Walsh RM Jr, Kim JJ, Hibbs RE. Structure of a human synaptic GABAA receptor. Nature. 2018 Jun 27; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Illinois
GABAA receptors, among the most predominant inhibitory receptors in the central nervous system, constitute heteromeric structures formed by the assembly of five subunits, the most common of which are α, β, γ, δ. These subunits host a number of binding sites for endogenous or synthetic modulators.
This work is an outstanding collection of data on the synaptic isoform of the human GABAA receptor. It sheds light into mechanisms by which the GABA neurotransmitter or endogenous modulators, neurosteroids (e.g., allopregnanolone) with selectivity for the β-α subunit interface, act on GABAA receptors.
Understanding the receptor architecture of GABAA receptors is pivotal not only to predict dysfunction of its homonymous neurotransmitter, GABA, but also for that of other endogenous modulators, including neurosteroids. Knowledge of its structure is also important to anticipate lack of pharmacological action of a number of other synthetic agents, including benzodiazepines, barbiturates, and anesthetics, and even alcohol.
Through their binding sites on the GABAA receptors, neurosteroids potentiate the action of GABA and play a neurophysiological role of fine-tuning of the receptor for synthetic modulators, including benzodiazepines and barbituates. Furthermore, neurosteroids, by regulating GABAA receptor strength, regulate emotional behaviors such as expression of aggression and impulsivity, anxiety, and fear. Hence, it is not surprising that this receptor is involved in neurological and psychiatric disorders that range from anxiety spectrum disorders to autism and from drug addiction to epilepsy and post-traumatic stress disorder (PTSD).
Both subunit expression and assembly of GABAA receptors, as well as the biosynthesis of GABA and neurosteroids, are highly susceptible to environmental factors, including stress and pharmacological treatment. Stress per se may change the expression of subunits such that the receptor becomes insensitive to the pharmacological action of benzodiazepines. For example, stress-induced changes in the neurobiology of PTSD result in a GABAA receptor conformation with reduced benzodiazepine binding sites. Indeed, benzodiazepines such as Xanax, which improve symptoms in anxiety disorders and panic, fail to help PTSD patients. Likewise, stress downregulates physiological levels of GABAergic neurosteroids, which results in decreased GABAergic neurotransmission and behavioral dysfunction.
In Alzheimer’s disease, both animal models and evidence from postmortem human brain suggest a number of morphological and functional changes consistent with remodeling of GABAA receptors.
For example, in AD brain areas, GABAA receptors were less sensitive to GABA, and GABAA receptor α1 and γ2 subunits were downregulated. Given that benzodiazepines bind at the interface of the α1-3,5/γ2, these changes are consistent with a lower efficacy of these anxiolytic drugs, which may prove unsuccessful in controlling behavioral changes in AD patients, including anxiety and impulsivity.
Hence, the work presented by Zhu and colleagues will help us understand why current drugs fail to exert pharmacological properties, but most importantly, it will help anticipate future design of successful GABAA receptor agents for the treatment of neurological and psychiatric disorders.
Make a Comment
To make a comment you must login or register.