Can a Fatty Acid Explain Sex Differences in Parkinson’s?
Quick Links
Women are less likely to get Parkinson’s disease than men, and scientists suspect this may be thanks to estrogen. But how? By binding estrogen receptor α (ERα) at synapses and rendering α-synuclein more soluble, say Silke Nuber at Brigham and Women’s Hospital, Boston, and colleagues. Counterintuitively, male and female mice have similar levels of estrogen receptors in their neurons, but in a synucleinopathy model, males have less at their synapses, the authors report in the November 17 Science Advances.
- In Parkinson’s model, females have milder pathology and symptoms.
- Estrogen drives this protection by binding estrogen receptor a at the synaptic membrane.
- In males, ERα strays from the synapse.
- Stabilizing ERα palmitoylation restored cognition and motor skills.
The deficit stems from poor palmitoylation, which glues the receptor to the membrane. Without ERα, synaptic vesicles stall, letting α-synuclein aggregate. A small molecule that blocks de-palmitoylation retained the receptor at the synapse surface, reduced α-synuclein toxicity, and normalized memory and motor symptoms.
“The underlying causes of sex differences in PD susceptibility are something of a black box, and these studies are headed in the right direction,” wrote Subhojit Roy, University of California, San Diego (comment below). “The experiments are technically superb, and the data are clear. This is a nice study opening up a field where few mechanistic studies exist.”
Women are half as likely to develop PD as men, and tend to have milder disease (Reekes et al., 2020; Oltra et al., 2021). Nuber had seen a similar difference in 3K mice, which overexpress human α-synuclein with three lysine mutations linked to familial PD (Oct 2018 news). Females had less synucleinopathy and milder motor symptoms than males. Giving 3K males 10β,17β-dihydroxyestra-1,4-dien-3-one (DHED), a molecule that cells metabolize to estrogen only in the brain, reduced α-synuclein accumulation and lengthened stays on a rotarod (Rajsombath et al., 2019).
To understand how estrogen protects, first author Tim Moors studied a milder version of the 3K mice, dubbed “3KL” for its lower expression level of the offending α-synuclein gene. Just as in 3K mice, 3KL females were healthier than males. The latter began to have trouble coordinating movement and recognizing new objects at 6 months, but the former stayed sprightly until 1 year of age. Again, DHED helped the males, stirring their synapses to fire just like wild-types. To figure out which estrogen receptor might be involved, the scientists bathed hippocampal slices in selective inhibitors of ERα or ERβ. The former, but not the latter, blocked DHED effects.
3KL mice had as many ERα receptors as controls; alas, their distribution differed. Rather than flocking to the synaptic surface to help shuttle vesicles, as they usually do, in males the receptors co-localized with α-synuclein aggregates.
Looking in two postmortem samples from people, Moors spotted ERα in Lewy bodies within the substantia nigra and hippocampal tissue from a man who had had PD and a woman who'd had dementia with Lewy bodies.
What about sex differences? Again, male 3KL mice had as many estrogen receptors within neurons as did females, but because males had larger α-synuclein aggregates, fewer receptors reached the synaptic membrane.
Nuber and colleagues think this shortfall of synaptic ERα spurs a vicious pathological cycle. Within the bustling environment of the synapse, α-synuclein monomers bind to vesicles, twist into α-helices, then dissociate and form aggregate-resistant tetramers. If monomers linger on vesicles instead, they are more likely to aggregate (Apr 2015 conference news; Oct 2016 news). Nuber believes this happens when there is too little synaptic ERα to help ferry vesicles: their turnover stalls, as does release of α-synuclein monomers.
Why did ERα drift away from the synaptic membranes to begin with? Nuber suspected a lack of palmitoylation, a covalent attachment of palmitic acid to cysteine and amide residues in proteins (image below). Since the fatty acid can insinuate into lipid bilayers, it helps anchor proteins to membranes (reviewed by Main and Fuller, 2021). In the case of ERα, palmitoylation modulates where it localizes in the cell (reviewed by Marino et al., 2006). /papers/s-palmitoylation-modulates-estrogen-receptor-alpha-localization-and-functions Indeed, both male and female 3KL mice had less palmitoylated ERα than did wild-types, as measured by a chemical assay. Acyl-biotin exchange swaps palmitate from modified cysteines with biotin, which can be measured by western blot (Brigidi and Bamji, 2013).
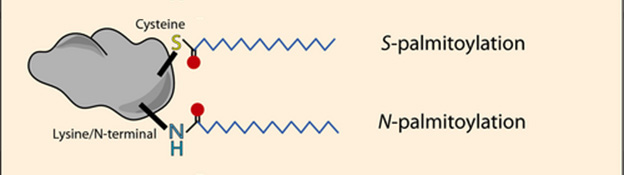
Fattened Up. Palmitoylation adds palmitic acid to cysteine and/or amide groups on proteins. [Modified from Main and Fuller, 2021].
Since palmitoylation is reversible, the scientists wondered if blocking de-palmitoylation of ERα would keep it at synapses longer. Moors fed 3KL mice ML348, an inhibitor of APT1, a thioesterase that removes palmitic acid from proteins. After 90 days, females and males had 50 percent more palmitoylated ERα at the synaptic membrane, as determined by proximity ligation assay. They also had fewer α-synuclein aggregates, and a higher ratio of synuclein tetramers to monomers in their hippocampi (image below). ML348 enabled mice to stay on a rotating beam for longer and more accurately distinguish new objects from old ones.
“[These results] advance [the] understanding of the intimate connection between α-synuclein conformation (multimers), solubility, and localization with PD phenotypes, highlighting the importance of this aspect of α-synuclein biology in PD,” wrote Saranna Fanning, who heads a separate lab at Brigham and Women’s but was not involved in this study (comment below).

Please Palmitoylate Me. The hippocampi and cortices of female (top left) 3KL mice had fewer α-synuclein aggregates than males (bottom left). Both sexes had fewer aggregates after getting the APT1 inhibitor ML348 (right slices). [Courtesy of Moors et al., Science Advances, 2023.]
To Nuber's mind, finding ways to maintain a physiological α-synuclein tetramer-to-monomer ratio, such as preserving ERα palmitoylation, might lead to new therapeutics for early PD. To Nagendran Ramalingam, also at Brigham and Women’s but not involved in this study, it’s early days yet. “While the results are encouraging, and modulating synaptic function is a potential disease-modifying therapy, we have to wait and see the therapeutic value of palmitoylated ERα,” he wrote (comment below).—Chelsea Weidman Burke
References
News Citations
- Sans Synuclein Tetramers, Mice Mimic Parkinson’s Disease
- Form and Function: What Makes α-Synuclein Toxic?
- Fatty Acid Greases the Wheels for α-Synuclein Multimers
Paper Citations
- Reekes TH, Higginson CI, Ledbetter CR, Sathivadivel N, Zweig RM, Disbrow EA. Sex specific cognitive differences in Parkinson disease. NPJ Parkinsons Dis. 2020;6:7. Epub 2020 Apr 8 PubMed.
- Oltra J, Segura B, Uribe C, Monté-Rubio GC, Campabadal A, Inguanzo A, Pardo J, Marti MJ, Compta Y, Valldeoriola F, Iranzo A, Junque C. Sex differences in brain atrophy and cognitive impairment in Parkinson's disease patients with and without probable rapid eye movement sleep behavior disorder. J Neurol. 2021 Aug 3; PubMed.
- Rajsombath MM, Nam AY, Ericsson M, Nuber S. Female Sex and Brain-Selective Estrogen Benefit α-Synuclein Tetramerization and the PD-like Motor Syndrome in 3K Transgenic Mice. J Neurosci. 2019 Sep 18;39(38):7628-7640. Epub 2019 Aug 12 PubMed.
- Main A, Fuller W. Protein S-Palmitoylation: advances and challenges in studying a therapeutically important lipid modification. FEBS J. 2022 Feb;289(4):861-882. Epub 2021 Mar 18 PubMed.
- Brigidi GS, Bamji SX. Detection of protein palmitoylation in cultured hippocampal neurons by immunoprecipitation and acyl-biotin exchange (ABE). J Vis Exp. 2013 Feb 18;(72) PubMed.
Further Reading
Primary Papers
- Moors TE, Li S, McCaffery TD, Ho GP, Bechade PA, Pham LN, Ericsson M, Nuber S. Increased palmitoylation improves estrogen receptor alpha-dependent hippocampal synaptic deficits in a mouse model of synucleinopathy. Sci Adv. 2023 Nov 15;9(46):eadj1454. Epub 2023 Nov 17 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of California, San Diego
Underlying causes of sex differences in PD susceptibility is something of a black box, and these studies are headed in the right direction. The experiments are technically excellent, and the data are very clear.
More studies focused on this important problem are needed. One issue is that it is not clear if gender differences in PD models that genetically overexpress WT or mutant α-synuclein are relevant in the context of sporadic Parkinson’s disease, where these gender difference are actually seen. However, there is no easy way to address these questions.
Harvard Medical School and Brigham & Women's Hospital
This publication builds on Silke Nuber’s work on sex differences in PD in vivo models (Rajsombath et al., 2019), and that of Dennis Selkoe and Gary Ho on palmitoylation modifying αS PD phenotypes including neurotoxicity and αS accumulation (Ho et al., 2021; Ho et al., 2023).
The new paper makes several important contributions to the field of PD. It progresses our understanding of differences in PD in females and males in vivo. It is an exciting advance in determining an early αS-induced synaptic impairment, partly contributed by palmitoylated ERα. It furthers mechanistic insight on synaptic changes in males versus females and supports palmitoylation modification as a candidate therapeutic approach.
The group discerns an abnormal distribution of ERα in 3KL mice associated with reduced palmitoylation. Treatment with the APT1 inhibitor ML348 improved aberrant cognitive and motor phenotypes, correlating with restoring synaptic plasticity. Of particular interest is the correlation of cognitive function in 6-month-old 3KL females with increased αS multimers and αS solubility. This advances understanding of the intimate connection between αS conformation (multimers), αS solubility, and αS localization with PD phenotypes in vivo, highlighting the importance of this aspect of αS biology in PD.
References:
Rajsombath MM, Nam AY, Ericsson M, Nuber S. Female Sex and Brain-Selective Estrogen Benefit α-Synuclein Tetramerization and the PD-like Motor Syndrome in 3K Transgenic Mice. J Neurosci. 2019 Sep 18;39(38):7628-7640. Epub 2019 Aug 12 PubMed.
Ho GP, Ramalingam N, Imberdis T, Wilkie EC, Dettmer U, Selkoe DJ. Upregulation of Cellular Palmitoylation Mitigates α-Synuclein Accumulation and Neurotoxicity. Mov Disord. 2021 Feb;36(2):348-359. Epub 2020 Oct 26 PubMed.
Ho GP, Wilkie EC, White AJ, Selkoe DJ. Palmitoylation of the Parkinson's disease-associated protein synaptotagmin-11 links its turnover to α-synuclein homeostasis. Sci Signal. 2023 Feb 14;16(772):eadd7220. PubMed.
Brigham & Women's Hospital/Harvard Medical School
This is elegant work by my colleagues Silke Nuber, Tim Moors, and team. It combines sexual dimorphism and protein palmitoylation to reinforce the view that synaptic dysfunction is one of the key drivers of early synuclein pathology. While the results are encouraging, and modulating synaptic function is a potential disease-modifying therapy, we have to wait and see the therapeutic value of palmitoylated estrogen receptor-α (ER-α).
Some of the open questions that the team may already be addressing include:
1) whether ER-α palmitoylation is reduced in Parkinson’s disease and Lewy body dementia patients,
2) what does it mean that in early pathology, ER-α palmitoylation appears to be not different between male and female 3K-low mice,
3) what is the functional relevance of palmitoylated ER-α in restoring synaptic deficits in synucleinopathy.
As the Nuber team points out, the latter may particularly be challenging because of the lack of feasible options, but it will be important.
Harvard Medical School, Brigham and Women’s Hospital
This is a thought-provoking study by my colleagues at BWH. Moors et al. build on the lab’s previous insight into how female sex may protect from α-synuclein pathology. Digging deeper into the mechanism(s), this paper identifies estrogen-receptor α as a key player.
Previous work by other groups has shown that estrogen-receptor α is found at synapses, attached to synaptic vesicles via a palmitoyl anchor.
It seems to have a positive effect on synaptic transmission, possibly by increasing the mobility of synaptic vesicles.
In that role, estrogen-receptor α may have two beneficial effects on αS-related pathology: (1) it counteracts the vesicle accumulation/aggregation that seems to be triggered by α-synuclein excess at membranes; (2) it helps get α-synuclein off the vesicle membranes. Unsurprisingly, both roles seem to depend on proper palmitoylation. This offers a regulatory step that, who knows, may become druggable by specific compounds (the ML438 drug used in the study is not specific to estrogen-receptor α palmitoylation).
However, while estrogen-receptor α seems to be a good influence on α-synuclein, α-synuclein seems to be a bad influence on estrogen-receptor α: excess membrane α-synuclein may interfere with estrogen-receptor α palmitoylation, leading to mislocalization and loss of function.
As the authors correctly point out, the intriguing findings from the “3K” mouse model of α-synuclein membrane excess will need to be tested in other models, including models of α-synuclein cytosolic accumulation or seeded α-synuclein aggregation. Different outcomes in such models, however, would not necessarily question the role of estrogen-receptor α in membrane-mediated α-synuclein toxicity. It could also help differentiate between different pathways to α-synuclein toxicity, identifying the respective key players. It looks like estrogen-receptor α may very well be such a player, at least in a subset of synucleinopathies.
Make a Comment
To make a comment you must login or register.