Blood May Foretell Parkinson’s Up to Seven Years Prior
Quick Links
Clinicians are in desperate need of fluid markers that can detect Parkinson’s disease at the earliest stages. Now, they may have a candidate. In the June 18 Nature Communications, scientists led by Brit Mollenhauer at University Medical Center Göttingen, Germany, and Kevin Mills at University College London, reported a panel of eight plasma proteins that cleanly distinguished people with Parkinson’s from healthy controls in a small cohort. Among people with REM sleep behavior disorder, a common precursor of PD, the panel also predicted who would develop motor symptoms within the next seven years.
- A panel of eight plasma proteins distinguished people with Parkinson’s from controls.
- The proteins implicate neuroinflammation, cellular stress, and waning neuroprotection.
- People tested positive up to seven years before motor symptoms.
If the findings hold up in larger cohorts, the assay could help select participants for prevention trials, noted co-first author Michael Bartl at Göttingen.
Jarod Rutledge at Stanford University, Palo Alto, California, called the data an advance over previous preclinical biomarker studies, which did not follow participants long enough to demonstrate progression to motor symptoms. “Importantly, this is the first concrete demonstration that markers in blood can predict Parkinson’s onset in presymptomatic patients at high risk,” he wrote to Alzforum (comment below).
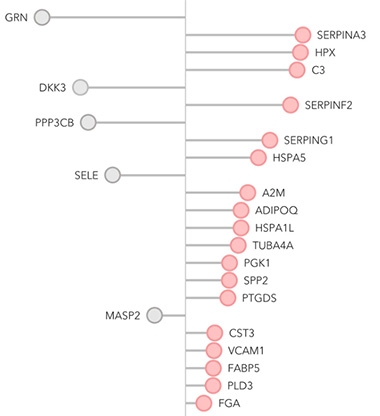
Parkinson’s Signature? In the discovery phase, researchers identified 23 plasma proteins associated with the disease; 18 were elevated (red) and five suppressed (gray). [Courtesy of Hällqvist et al., 2024.]
To date, the most promising PD biomarkers are α-synuclein seed amplification assays, which test for the presence of fibrils in cerebrospinal fluid or skin (Apr 2023 conference news; Aug 2023 conference news; Apr 2024 news). Clinicians prefer blood tests, however, and here the main biomarker is an uptick in neurofilament light, which is not specific for PD (Jun 2016 news; Feb 2017 news).
To identify blood biomarkers that would flag PD reliably at early stages, joint first authors Bartl and Jenny Hällqvist at UCL analyzed plasma samples from 10 people with PD and 10 age-matched controls using an unbiased mass spectrometry approach. This turned up 47 differentially expressed proteins. Next, they tested these, along with a suite of known Parkinson’s and inflammatory markers. Of the 121 total plasma proteins in this set, 23 were consistently different between 99 PD patients and 36 age-matched controls from a second cohort (image above).
To winnow down the panel, the authors used machine learning. Analyzing data from 70 percent of the cohort, this approach picked out a set of eight proteins, seven of which had been in the set of 23. In testing on the remaining 30 percent of the cohort, the panel accurately separated patients from controls. Consistently, Parkinson’s patients had high levels of complement protein C3, serine protease inhibitor G1, heat shock protein A5, prostaglandin-D2 synthase, and intercellular adhesion molecule 1, along with low levels of progranulin, dickkopf WNT signaling pathway inhibitor 3, and mannan-binding serine peptidase 2. Bartl told Alzforum the next step will be to validate this panel in an independent cohort.
The authors believe these proteins point toward interesting biology. C3, SerpinG1, and MASP2 all take part in the complement cascade, an innate immune pathway linked to neurodegenerative disease. Heat shock proteins are involved in cellular stress responses and protein folding. Progranulin and PTGDS are neuroprotective proteins, and WNT signaling helps maintain dopaminergic neurons (for review, see Arenas, 2014). Together, the findings highlight the role of inflammation, cellular stress, and waning neuroprotection in PD pathogenesis, the authors noted.
Moreover, patients with the greatest changes in these proteins also had the worst cognitive and motor scores, hinting at biological relevance. Nonetheless, the authors cautioned that this is a peripheral disease signature that may not directly reflect what is happening in the brain, since plasma profiles correlated poorly with CSF samples from the same patients.
To find out how early this eight-protein panel could detect disease, the authors tested it in a cohort of 54 people with isolated REM sleep behavior disorder. About 80 percent of people with iRBD eventually develop synucleinopathy (Sep 2013 news; for review see Hu, 2020). Participants were followed for up to 10 years and donated an average of three blood samples each. The protein panel identified 79 percent of the samples as PD-positive, in line with the expected percentage of synucleinopathy.
Eleven of these iRBD patients developed Parkinson’s, and five developed dementia with Lewy bodies. Eight of the PD cases, and two of the DLB cases, had tested positive up to seven years before motor symptoms developed.
“By the time Parkinson’s patients have motor symptoms, 70 to 80 percent of their dopaminergic neurons are gone,” Bartl told Alzforum. “We need to treat earlier.” He believes this plasma assay could be a good starting point for pinpointing those prodromal patients. In future work, the authors will test the panel in people at even earlier stages of PD, such as those who have lost their sense of smell, and in a population-based aging study in Kassel, Germany. They will also test it in other synucleinopathies such as DLB and multiple system atrophy.—Madolyn Bowman Rogers
References
News Citations
- Synuclein Assay Passes the Sniff Test—What of Other Seeds?
- Finally, a Diagnostic Marker for Lewy Body Disease?
- Skin Deep: Punch Biopsies Detect Synucleinopathies
- Blood NfL Looks Good as Progression and Outcome Marker
- Touchdown for NfL: Blood Test Tells Parkinson's from Related Disorders
- Non-Motor Symptoms Are an Early Sign of Parkinson's
Paper Citations
- Arenas E. Wnt signaling in midbrain dopaminergic neuron development and regenerative medicine for Parkinson's disease. J Mol Cell Biol. 2014 Feb;6(1):42-53. Epub 2014 Jan 14 PubMed.
- Hu MT. REM sleep behavior disorder (RBD). Neurobiol Dis. 2020 Sep;143:104996. Epub 2020 Jun 26 PubMed.
Further Reading
News
- Methods to Detect Amyloid Seeds Improve, Extend to Blood and Parkinson’s
- Biomarkers Differentiate Parkinsonian Diseases and Forecast Decline
- Neurodegenerative Proteins in the Skin Could be Diagnostic
- Fluid Biomarkers Give Clues to Disease Progression in Parkinson’s
- Thinning Retina … Could It Be Parkinson’s?
- Spitting, Sniffing: Is This How We Will Dx Parkinson’s?
- Meet the Two New Biomarker Candidates for Lewy Body Diseases
- A Biological Definition of Parkinson’s Disease: Two Proposals Pave the Way
Primary Papers
- Hällqvist J, Bartl M, Dakna M, Schade S, Garagnani P, Bacalini MG, Pirazzini C, Bhatia K, Schreglmann S, Xylaki M, Weber S, Ernst M, Muntean ML, Sixel-Döring F, Franceschi C, Doykov I, Śpiewak J, Vinette H, Trenkwalder C, Heywood WE, Mills K, Mollenhauer B. Plasma proteomics identify biomarkers predicting Parkinson's disease up to 7 years before symptom onset. Nat Commun. 2024 Jun 18;15(1):4759. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
The Amaranth Foundation
This study adds to growing evidence that protein biomarkers in biofluids can differentiate people with PD from healthy individuals, potentially before symptoms appear (Pereira et al., 2023; Virreira Winter et al., 2021; Rutledge et al., 2024). While the claim of 100 percent sensitivity and specificity is very likely a result of methodological limitations, this study nevertheless lends additional weight to the idea that small protein panels could be effective for PD screening and diagnosis (Rutledge et al., 2022; Del Campo et al., 2023), extending this further than previous work. Importantly, this is the first concrete demonstration that markers in blood can predict PD onset in presymptomatic patients at high risk.
A particularly interesting piece of the study is the use of a longitudinally sampled iRBD cohort with a decade of follow-up, providing rare and valuable data. Approximately 80 percent of these samples were identified as PD, aligning with expected progression rates, and importantly, presymptomatic PD detection was achieved in 16/16 cases that progressed to motor symptoms over the study period. While other biomarker studies have reported similar results in presymptomatic cases, they have lacked long enough follow-up to concretely show biomarkers predict progression. I expect this new evidence will have an important impact on clinical translation of these ideas.
The study also employs a targeted mass spectrometry approach for biomarker analysis, rather than affinity-based methods such as Olink and SomaScan used in other reports. The authors also demonstrate translation of findings from plasma to serum, something that would likely be difficult for affinity proteomics. This may highlight an advantage of mass spec in the screening use case.
Despite high reported sensitivity and specificity, real-world replication is doubtful. The authors did not use independent train and test datasets to arrive at these numbers. Indeed, using cross-validation they reported precision and recall of 87 percent, and a balanced accuracy of 82 percent. This puts the accuracy of the test in a similar range to that of the single protein marker DOPA decarboxylase, as recently reported by us and others (Pereira et al., 2023; Rutledge et al., 2024; Rutledge et al., 2022; Del Campo et al., 2023; Paslawski et al., 2023). Cross-validation is almost always an overestimate of the test performance compared to verification using independent data, so it is highly likely the real accuracy is lower than reported.
References:
Pereira JB, Kumar A, Hall S, Palmqvist S, Stomrud E, Bali D, Parchi P, Mattsson-Carlgren N, Janelidze S, Hansson O. DOPA decarboxylase is an emerging biomarker for Parkinsonian disorders including preclinical Lewy body disease. Nat Aging. 2023 Oct;3(10):1201-1209. Epub 2023 Sep 18 PubMed.
Virreira Winter S, Karayel O, Strauss MT, Padmanabhan S, Surface M, Merchant K, Alcalay RN, Mann M. Urinary proteome profiling for stratifying patients with familial Parkinson's disease. EMBO Mol Med. 2021 Mar 5;13(3):e13257. Epub 2021 Jan 22 PubMed.
Rutledge J, Lehallier B, Zarifkar P, Losada PM, Shahid-Besanti M, Western D, Gorijala P, Ryman S, Yutsis M, Deutsch GK, Mormino E, Trelle A, Wagner AD, Kerchner GA, Tian L, Cruchaga C, Henderson VW, Montine TJ, Borghammer P, Wyss-Coray T, Poston KL. Comprehensive proteomics of CSF, plasma, and urine identify DDC and other biomarkers of early Parkinson's disease. Acta Neuropathol. 2024 Mar 11;147(1):52. PubMed.
Rutledge J, Lehallier B, Zarifkar P, Losada PM, Ryman S, Yutsis M, Deutsch G, Mormino E, Trelle A, Wagner AD, Kerchner GA, Tian L, Henderson VW, Montine TJ, Borghammer P, Wyss-Coray T, Poston KL. Aromatic L-Amino Acid Decarboxylase is a novel fluid biomarker of Parkinson's disease. 2022 Nov 11 10.1101/2022.11.09.22282149 (version 1) medRxiv.
Del Campo M, Vermunt L, Peeters CF, Sieben A, Hok-A-Hin YS, Lleó A, Alcolea D, van Nee M, Engelborghs S, van Alphen JL, Arezoumandan S, Chen-Plotkin A, Irwin DJ, van der Flier WM, Lemstra AW, Teunissen CE. CSF proteome profiling reveals biomarkers to discriminate dementia with Lewy bodies from Alzheimer´s disease. Nat Commun. 2023 Sep 13;14(1):5635. PubMed.
Paslawski W, Khosousi S, Hertz E, Markaki I, Boxer A, Svenningsson P. Large-scale proximity extension assay reveals CSF midkine and DOPA decarboxylase as supportive diagnostic biomarkers for Parkinson's disease. Transl Neurodegener. 2023 Sep 4;12(1):42. PubMed.
Banner Sun Health Research Institute
As usual for biomarker studies “validated” by the clinical diagnosis of PD, the “true” diagnostic accuracy cannot be claimed to be higher than the known clinical diagnostic accuracy against the postmortem diagnosis of PD. As these are all “de novo” PD cases and most of the cases, at least in the supplemental table 1, are of less than three years disease duration, and as the diagnostic accuracy against the postmortem neuropathological diagnosis of PD for such early PD cases that we and others have published is only between 60-70 percent at most (Beach et al., 2023; Adler et al., 2021; Beach and Adler, 2018; Adler et al., 2014), then this new diagnostic test’s true accuracy for PD cannot be any greater than that. Additionally, there did not seem to be third-party blinding (triple-blinding) so there is a potential for conscious or subconscious bias.
References:
Beach TG, Adler CH, Shill HA, Zhang N, Driver-Dunckley ED, Mehta SH, Serrano GE. Accuracy of the Early Diagnosis of Parkinson's Disease. Mov Disord. 2023 Aug;38(8):1573-1574. PubMed.
Adler CH, Beach TG, Zhang N, Shill HA, Driver-Dunckley E, Mehta SH, Atri A, Caviness JN, Serrano G, Shprecher DR, Sue LI, Belden CM. Clinical Diagnostic Accuracy of Early/Advanced Parkinson Disease: An Updated Clinicopathologic Study. Neurol Clin Pract. 2021 Aug;11(4):e414-e421. PubMed.
Beach TG, Adler CH. Importance of low diagnostic Accuracy for early Parkinson's disease. Mov Disord. 2018 Oct;33(10):1551-1554. Epub 2018 Oct 4 PubMed.
Adler CH, Beach TG, Hentz JG, Shill HA, Caviness JN, Driver-Dunckley E, Sabbagh MN, Sue LI, Jacobson SA, Belden CM, Dugger BN. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 2014 Jul 29;83(5):406-12. Epub 2014 Jun 27 PubMed.
University of Pennsylvania, Veterans Affairs Medical Center
This biomarker study establishes a novel, mass-spectrometry-based paradigm to identify, in an unbiased manner, biomarker proteins in blood that differentiate healthy individuals from those with Parkinson’s disease. Within this study, a panel of eight proteins shows perfect identification of individuals with Parkinson’s disease relative to healthy controls with strong cross-validation metrics. As with all biomarker studies, replication of these protein markers in an independent cohort, as well as comparison with other established biomarkers for Lewy body diseases, including skin biopsy immunofluorescence (Gibbons et al., 2024) and cerebrospinal fluid seed aggregation assay (Siderowf et al., 2023), will determine the real-world clinical and diagnostic utility of the identified proteins.
Moreover, the findings are exciting because they implicate a biochemical signature in individuals with REM-behavior disorder (RBD) that is not directly tied to α-synuclein pathology or dopamine metabolism, yet is present before phenoconversion. Despite less than 80 percent of individuals with RBD in this study having this signature, it was longitudinally stable in those who did. While this biomarker signature did not reliably predict, or correlate with, phenoconversion to neurodegeneration, understanding its mechanistic relevance in individuals with RBD, and its presence before onset of PD, will be very important to the field. Notably, this biochemical signature reinforces the idea of a dysregulated inflammatory state in individuals with, and at risk for, Lewy body diseases.
References:
Gibbons CH, Levine T, Adler C, Bellaire B, Wang N, Stohl J, Agarwal P, Aldridge GM, Barboi A, Evidente VG, Galasko D, Geschwind MD, Gonzalez-Duarte A, Gil R, Gudesblatt M, Isaacson SH, Kaufmann H, Khemani P, Kumar R, Lamotte G, Liu AJ, McFarland NR, Miglis M, Reynolds A, Sahagian GA, Saint-Hillaire MH, Schwartzbard JB, Singer W, Soileau MJ, Vernino S, Yerstein O, Freeman R. Skin Biopsy Detection of Phosphorylated α-Synuclein in Patients With Synucleinopathies. JAMA. 2024 Apr 16;331(15):1298-1306. PubMed.
Siderowf A, Concha-Marambio L, Lafontant DE, Farris CM, Ma Y, Urenia PA, Nguyen H, Alcalay RN, Chahine LM, Foroud T, Galasko D, Kieburtz K, Merchant K, Mollenhauer B, Poston KL, Seibyl J, Simuni T, Tanner CM, Weintraub D, Videnovic A, Choi SH, Kurth R, Caspell-Garcia C, Coffey CS, Frasier M, Oliveira LM, Hutten SJ, Sherer T, Marek K, Soto C, Parkinson's Progression Markers Initiative. Assessment of heterogeneity among participants in the Parkinson's Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 2023 May;22(5):407-417. PubMed.
University of Cambridge
The best treatment for Parkinson’s Disease (PD) would be to prevent the loss of existing dopaminergic neurons, but it is challenging to identify individuals who would benefit from such an intervention. This study from Hällqvist and colleagues is an important step in this direction, since they can distinguish people who are undergoing neurodegeneration, but have not yet developed PD-associated motor symptoms.
One potential criticism of the study is that there are many different types of neurodegenerative diseases, and they only compare samples from PD patients to a few other cohorts, so it’s unclear how specific their panel of biomarkers is for PD. However, if these biomarkers are also altered in other neurodegenerative conditions, and there are neuroprotective treatments that target shared mechanisms between those diseases, then the potential impact of their findings extends beyond just PD.
Make a Comment
To make a comment you must login or register.