Beyond Microglia—Alzheimer’s Gene PLCγ2 Acts on Synapses, Too.
Quick Links
The Alzheimer’s risk gene phospholipase C-γ2 has been thought to act mainly in microglia. Now, in a May 1 preprint on bioRxiv, researchers led by Jean-Charles Lambert, Julie Dumont, and Julien Chapuis at Institut Pasteur de Lille, France, Mikko Hiltunen at the University of Eastern Finland, Kuopio, and Christophe Mulle at the University of Bordeaux, France, challenge this idea by showing that PLCγ2 also affects synaptic health. In mice and cultured human neurons, knocking down PLCγ2 decreased synapse number and suppressed neuronal excitability. Knockdown also revved up Aβ production and tau phosphorylation in neurons. Tellingly, the authors correlated several rare, loss-of-function variants in PLCγ2 with a 10-fold higher chance of getting late-onset Alzheimer’s disease, highlighting the gene’s dramatic effect. That makes these variants stronger risk factors than a single ApoE4 allele, or TREM2 and most SORL1 variants. Lambert noted that to his knowledge, this is the first time PLCγ2 LoF variants have been associated with AD risk.
- Knockdown of AD gene PLCγ2 curtails synapses and neuronal excitability.
- Levels of Aβ and p-tau creep up.
- Very rare loss-of-function variants increase a person’s risk of AD 10-fold.
The data suggest that PLCγ2 has pleotropic effects in AD, acting on multiple cell types, Lambert and Dumont told Alzforum. “This makes it a particularly important gene for understanding the etiology of the disease, and also for developing therapeutic approaches,” they wrote. PLCγ2 activators are being developed as potential AD treatments (e.g., Visvanathan et al., 2023).
Arne Ittner at Flinders University, Adelaide, Australia, called the findings exciting. “The new paper provides strong evidence for a cell-autonomous function of PLCγ2 in neurons,” he wrote. However, he cautioned that before trying to activate PLCγ2 therapeutically, the gene’s physiological functions in neurons, microglia, and other cell types such as blood cells, where it is highly expressed, need to be better understood (comment below).
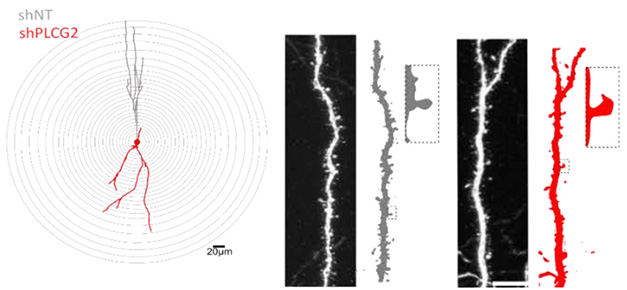
Synapse Loss. In mouse hippocampus, dendrites (gray) are shorter (left) after PLCg2 knockdown (red) and spines (right) are sparse and misshapen. [Courtesy of Coulon et al., bioRxiv.]
What’s known so far? The transmembrane PLCγ2 snips a membrane lipid, phosphatidylinositol-4,5-bisphosphate (PIP2), to form intermediates that trigger several downstream signaling cascades. It was first linked to AD in 2017, when geneticists identified a hyperactive variant, P522R, that lowered risk and was associated with healthy aging (Aug 2017 conference news; May 2019 news). PLCγ2 is highly expressed in microglia, where it stimulates beneficial activation, phagocytosis, and plaque compaction (Jun 2020 news; Sep 2020 news). Meanwhile, a hypomorphic variant, M28L, dampens microglial activation and amplifies AD risk (Sep 2023 news).
Lambert and colleagues became interested in PLCγ2 while screening for AD genes that affect synapses. In rat hippocampal primary neurons, joint first authors Audrey Coulon and Florian Rabiller at the Pasteur Institute, Mari Takalo and Henna Martiskainen in Kuopio, and Avishek Roy in Bordeaux knocked down 198 genes linked to 76 GWAS loci, then evaluated any synaptic changes (Feb 2021 news; Apr 2022 news). Five genes lowered synaptic density and four raised it.
The researchers focused on PLCγ2, because the lipase resides in both pre- and post-synapses, and its absence suppressed firing in the rat neurons. To further characterize its electrophysiological effects, they knocked down PLCγ2 in the dentate gyrus of adult wild-type mice. This thinned out the number of dendritic spines by two-thirds, while halving their volume. In hippocampal slice cultures, lowering PLCγ2 dampened neuronal excitability, suppressing action potentials and excitatory post-synaptic currents.
The authors repeated the experiments in neurons generated from human iPS cells. In these neurons, not only did PLCγ2 knockdown lower excitability, it also boosted the amount of Aβ precursor protein and tau protein by about half. The cells made three times as much Aβ1-x as controls, and had about twice as much of several types of phosphorylated tau, including at amino acids 181, 217, and 231. Notably, knockdown increased activation of the downstream kinases AKT and GSK3β, suggesting the lipase suppresses them.
Does PLCγ2 have the same effects in human brain? To investigate, the authors mined two large whole-exome datasets, the Finnish ADGEN and European ADES cohorts, comprising 9,259 AD cases and 17,662 controls (Bis et al., 2018; Holstege et al., 2022). They identified nine rare loss-of-function variants in PLCγ2, four of which had never been described before. These LoF variants were 10-fold more common in AD cases than controls.
In future work, the authors are bringing together a consortium to better characterize PLCγ2 function in different brain cell types using transcriptomic and proteomic approaches.—Madolyn Bowman Rogers.
References
News Citations
- Searching for New AD Risk Variants? Move Beyond GWAS
- The Mutation You Want: It Protects the Brain, Extends Life
- Janus-Faced PLCγ2? Alzheimer’s Risk Protein Toggles TREM2 and TLR Pathways
- Protective AD Variant Pinpoints Sweet Spot for Microglial Activation
- PLCγ2 Variants Toggle Microglial Plaque Compactors
- Massive GWAS Meta-Analysis Digs Up Trove of Alzheimer’s Genes
- Paper Alert: Massive GWAS Meta-Analysis Published
Paper Citations
- Visvanathan R, Utsuki T, Beck DE, Lendy E, Sun KL, Liu Y, Hering KW, Mesecar A, Zhang ZY, Putt KS. A novel fluorogenic reporter substrate for 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase gamma-2 (PLCγ2): Application to high-throughput screening for activators to treat Alzheimer's disease. SLAS Discov. 2023 Jun;28(4):170-179. Epub 2023 Mar 17 PubMed.
- Bis JC, Jian X, Kunkle BW, Chen Y, Hamilton-Nelson KL, Bush WS, Salerno WJ, Lancour D, Ma Y, Renton AE, Marcora E, Farrell JJ, Zhao Y, Qu L, Ahmad S, Amin N, Amouyel P, Beecham GW, Below JE, Campion D, Charbonnier C, Chung J, Crane PK, Cruchaga C, Cupples LA, Dartigues JF, Debette S, Deleuze JF, Fulton L, Gabriel SB, Genin E, Gibbs RA, Goate A, Grenier-Boley B, Gupta N, Haines JL, Havulinna AS, Helisalmi S, Hiltunen M, Howrigan DP, Ikram MA, Kaprio J, Konrad J, Kuzma A, Lander ES, Lathrop M, Lehtimäki T, Lin H, Mattila K, Mayeux R, Muzny DM, Nasser W, Neale B, Nho K, Nicolas G, Patel D, Pericak-Vance MA, Perola M, Psaty BM, Quenez O, Rajabli F, Redon R, Reitz C, Remes AM, Salomaa V, Sarnowski C, Schmidt H, Schmidt M, Schmidt R, Soininen H, Thornton TA, Tosto G, Tzourio C, van der Lee SJ, van Duijn CM, Vardarajan B, Wang W, Wijsman E, Wilson RK, Witten D, Worley KC, Zhang X, Alzheimer’s Disease Sequencing Project, Bellenguez C, Lambert JC, Kurki MI, Palotie A, Daly M, Boerwinkle E, Lunetta KL, Destefano AL, Dupuis J, Martin ER, Schellenberg GD, Seshadri S, Naj AC, Fornage M, Farrer LA. Whole exome sequencing study identifies novel rare and common Alzheimer's-Associated variants involved in immune response and transcriptional regulation. Mol Psychiatry. 2018 Aug 14; PubMed.
- Holstege H, Hulsman M, Charbonnier C, Grenier-Boley B, Quenez O, Grozeva D, van Rooij JG, Sims R, Ahmad S, Amin N, Norsworthy PJ, Dols-Icardo O, Hummerich H, Kawalia A, Amouyel P, Beecham GW, Berr C, Bis JC, Boland A, Bossù P, Bouwman F, Bras J, Campion D, Cochran JN, Daniele A, Dartigues JF, Debette S, Deleuze JF, Denning N, DeStefano AL, Farrer LA, Fernández MV, Fox NC, Galimberti D, Genin E, Gille JJ, Le Guen Y, Guerreiro R, Haines JL, Holmes C, Ikram MA, Ikram MK, Jansen IE, Kraaij R, Lathrop M, Lemstra AW, Lleó A, Luckcuck L, Mannens MM, Marshall R, Martin ER, Masullo C, Mayeux R, Mecocci P, Meggy A, Mol MO, Morgan K, Myers RM, Nacmias B, Naj AC, Napolioni V, Pasquier F, Pastor P, Pericak-Vance MA, Raybould R, Redon R, Reinders MJ, Richard AC, Riedel-Heller SG, Rivadeneira F, Rousseau S, Ryan NS, Saad S, Sanchez-Juan P, Schellenberg GD, Scheltens P, Schott JM, Seripa D, Seshadri S, Sie D, Sistermans EA, Sorbi S, van Spaendonk R, Spalletta G, Tesi N, Tijms B, Uitterlinden AG, van der Lee SJ, Visser PJ, Wagner M, Wallon D, Wang LS, Zarea A, Clarimon J, van Swieten JC, Greicius MD, Yokoyama JS, Cruchaga C, Hardy J, Ramirez A, Mead S, van der Flier WM, van Duijn CM, Williams J, Nicolas G, Bellenguez C, Lambert JC. Exome sequencing identifies rare damaging variants in ATP8B4 and ABCA1 as risk factors for Alzheimer's disease. Nat Genet. 2022 Dec;54(12):1786-1794. Epub 2022 Nov 21 PubMed.
Further Reading
Primary Papers
- Coulon A, Rabiller F, Takalo M, Roy A, Martiskainen H, Siedlecki-Wullich D, Mendes T, Lemeu C, Carvalho L-I, Ehrardt A, MelodeFerias AR, Hulsman M, Najdek C, Lannette-Weimann N, Freire-Regatillo A, Amouyel P, Charbonnier C, Dols-Icardo O, Jeskanen H, Kuulasmaa T, Kurki M, Hardy J, Wagner M, Heikkinen S, Holstege H, Makinen P, Nicolas G, Mead S, Wagner M, Ramirez A, Rauramaa T, Palotie A, Sims R, Soininen H, vanSwieten J, Williams J, Willman R-M, Bellenguez C, Grenier-Boley B, Gelle C, Lambert E, Ayral A-. Neuronal downregulation of PLCG2 impairs synaptic function and elicits Alzheimer disease hallmarks. 2024 May 01 10.1101/2024.04.29.591575 (version 1) bioRxiv.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.