Two New p-Tau217 Blood Tests Join a Crowded Field
Quick Links
The blood-based p-tau marker field is getting pretty busy. In addition to existing tests by Fujirebio, Lilly, Janssen, and Meso Scale Discovery, C2N debuted a new CLIA-approved mass spectrometry-based test last August, while ALZPath Inc. still plans to offer its immunoassay for clinical use in 2023. Data presented at CTAD in Boston indicate that both new tests identify people with amyloid pathology with accuracies that rival the current crop of assays. A round-robin study, also shown at CTAD, suggests that plasma p-tau217 assays have the best chance of becoming stand-alone diagnostic tests for AD (see Part 11 of this series).
- C2N's new plasma test combines Aβ42/40 and p-tau217/tau217 ratios.
- ALZPath to offer p-tau217 immunoassay for clinical use this year.
- Both identify people with brain amyloid deposition.
PrecivityAD2, C2N’s new test, combined plasma Aβ42/40 and percentage of tau phosphorylated at position 217 to identify people who have 25 C2N’s centiloids or more on amyloid PET scans, which would include people with relatively low levels of amyloid pathology. At CTAD, C2N’s Tim West described how the test performed in two cohorts: 224 people in the Plasma Test for Amyloidosis Risk Screening study, aka PARIS, and 359 people in MissionAD, Eisai’s Phase 3 program for its discontinued BACE inhibitor elenbecestat.
The latter enrolled people with early AD. PARIS, on the other hand, is a sub-study of the Imaging Dementia–Evidence for Amyloid Scanning study. IDEAS recruited Medicare beneficiaries to test if amyloid imaging adds value in primary care settings (April 2015 news). “In terms of cohorts we can obtain samples from, this is as close as you can get to the real world,” said West. Since PARIS collected no blood samples, C2N sent phlebotomists to volunteers’ homes. “This worked out great,” West said. “I don’t think we could have recruited that many patients if they were required to go to a blood collection center.”
West had previously reported that the percentage of tau phosphorylated at 217 identified amyloid-positive people in PARIS with an AUC of 0.95 (Aug 2022 conference news). In Boston, West showed that for the 583 people in PARIS and MissionAD, the AUC for %p-tau217 was almost the same, 0.94.
C2N combined this tau marker with the Aβ42/40 ratio to predict the likelihood that a person's amyloid PET score surpasses 25 centiloids. West and colleagues determined that this so-called Amyloid Probability Score 2 had a sensitivity and specificity of 88 and 89 percent, respectively, in the PARIS/MissionAD cohorts.
This was better than the original APS score based on Aβ42/40, ApoE proteotype, and age. That earlier test had delivered a specificity of 86 percent, but only by excluding about 14 percent of people who fell into a no-man’s land, i.e., the intermediate zone where the APS score could not determine if they were indeed positive or negative for plaques. This was a problem with the test (image below). At CTAD, West showed that, in contrast, APS2 scores fell at the extreme ends of the range, i.e., well away from no man’s land, with an optimal cut point at 47.5 on a scale of 0 to 100. “With the new test, the prediction score is binary,” he told Alzforum. “People are either positive or negative.”
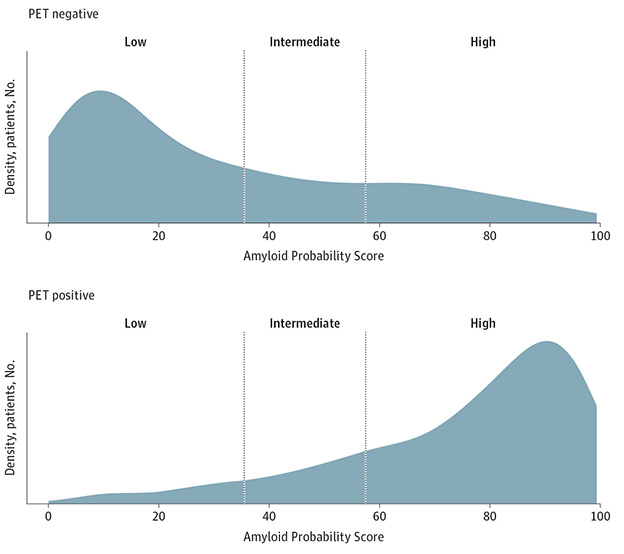
Better Precivity? In PrecivityAD, which was based on Aβ42/40, ApoE proteotype, and age, about 14 percent of people fell into an intermediate zone, meaning their Amyloid Probability Score (APS), failed to predict whether they were amyloid positive or negative. In PrecivityAD2 (not shown), APS2 scores concentrate at the extremes of the scale, allowing for a single cut point. [Courtesy of Hu et al., 2022].
How might the test perform in the real world? As with all tests, this depends on the prevalence of the disease, noted West. In a population where the dementia prevalence was 65 percent, PrecivityAD2 had positive and negative predictive values of 94 and 80 percent, respectively. In a population with mild cognitive impairment, where prevalence clocks in at around 50 percent, those numbers fell to 90 and 87 percent. These numbers seemed stable, unaffected by demographic variables, including race, ethnicity, sex, age, and ApoE4 status.
ApoE isoform, a test variable in the original PrecivityAD, is absent from PrecivityAD2. West told Alzforum that it didn’t budge the AUC enough to warrant including it. “And this way, we avoid having to disclose genetic information to people who take the test,” he added. Such revelations can worry some volunteers. C2N will continue to offer a separate ApoE test, West said. In 2019 it received Breakthrough Device Designation from the FDA for its amyloid blood tests, which cover PrecivityAD2, West told Alzforum, but the company has not filed for FDA approval yet. He said they have a set of prospectively collected PARIS samples on hold for this purpose.
A Different Path
For its part, Carlsbad, California-based ALZPath Inc. hopes to roll out its p-tau217 test for routine clinical use later this year. An immunoassay that uses proprietary antibodies and the highly sensitive single molecular array platform, aka SIMOA, ALZpath pTau217 is commercially available through Quanterix, a diagnostic company based in Billerica, Massachusetts.
Scientists have tested the ALZPath assay in more than 40 clinical cohorts worldwide. At CTAD, Hongming Zhang and colleagues from Frontage Laboratories, Exton, Pennsylvania, a contract research organization (CRO), reported on a validation study in which the assay had a lower limit of quantification of 9.77 fg/mL. Yes, that’s femtograms, and makes this test perhaps the most sensitive commercial p-tau217 assay. In a recent head-to-head comparison, which did not include the ALZpath test, the Janssen assay had a lower limit of detection of 13 fg/mL: Limits of detection are less stringent than limits of quantification (Janelidze et al., 2022). In Zhang’s hands, the minimal volume required—which can be in the mL range for some tests—was 33μL for plasma and 5μL for CSF. The variation between different runs of the same sample were low, indicating good precision.
Recently, scientists led by Kaj Blennow at University of Gothenburg, Sweden, reported that within-subject variation in the ALZpath assay was slightly worse than for Aβ42/40 assays, though not as bad as for p-tau181, but that the large fold change in p-tau217 between AD and control samples would balance out this variability (Brum et al., 2023).
At CTAD, Ahmed Chenna and colleagues at Labcorp-Monogram Biosciences, another CRO based in South San Francisco, reported that among 200 volunteers in the Australian Imaging, Biomarkers, and Lifestyle cohort, the ALZpath pTau217 plasma test best identified people who had amyloid in their brains per PET scan. It outperformed plasma p-tau181, Nf-L, GFAP, and Aβ42/40, returning an AUC of 0.95.
The next best was a Lumipulse assay of Aβ42/40 which rang in at 0.88. In a paper recently uploaded to medRxiv, scientists led by Nicholas Ashton at UGothenburg reported testing the ALZpath assay on samples from 786 participants in three cohorts, Translational Biomarkers in Aging and Dementia (TRIAD), Wisconsin Registry for Alzheimer’s Prevention, and the Sant Pau Initiative on Neurodegeneration. The assay identified amyloid pathology with AUCs of 0.92 to 0.96, and tangle pathology with slightly better accuracy, AUCs of 0.93 to 0.97 (Ashton et al., 2023).
Similarly, scientists at UGothenburg led by Andrea Benedet compared how the ALZpath and Janssen assays performed in the TRIAD cohort. The assays fared equally well, distinguishing people with AD from those with other neurodegenerative diseases with AUCs of O.95 and 0.96, respectively. The p-tau217 levels determined by both also correlated with amyloid and tangle PET SUVRs, though the Janssen assay tracked slightly better with tau PET than did the ALZpath one. Their preprint was uploaded to The Lancet on September 19 (Therriault et al., 2023).
In a separate study, scientists, including Jeromin, led by Ashley Price, Butler Hospital, Providence, Rhode Island, tested if ALZpath pTau217 and three other markers were able to identify people in preclinical stages of AD. They tested plasma from 143 cognitively healthy people, between the ages of 55 and 80, of whom 62 and 81 were deemed to have low and high risk, respectively, of getting AD. Eleven of the high-risk group carried one copy of APOE4, the other 70 had two copies.
First author Peter Snyder reported that only ALZpath pTau217 differentiated the low- and high-risk groups. Plasma Aβ40/42, NfL, and p-tau181 showed no difference. Among 25 people in the cohort who had amyloid scans, both Aβ40/42 and ALZpath pTau217 distinguished amyloid-positive from -negative, but the latter did so more robustly. The AUC in this population was 0.85. NfL, and p-tau181 correlated with age, but neither p-tau217 nor Aβ40/42 did, indicating these are less susceptible to “noise.” In fact, this might be why p-tau217 outperformed all other markers in the round-robin presented at CTAD. Other tau markers turn up in the plasma even in healthy controls.
As CTAD began, Quanterix announced it would partner with Janssen to commercialize Janssen’s p-tau217 assay. “This confused some of our customers,” ALZPath Inc’s Andreas Jeromin told Alzforum. “Quanterix will continue to support the ALZPath assay in parallel,” he assured Alzforum.—Tom Fagan
References
News Citations
- Plasma p-Tau-217 Assays Work Well, But No Home Run for Diagnosis
- $100M IDEAS: CMS Blesses Study to Evaluate Amyloid Scans in Clinical Practice
- Blood Tests Go Head-to-Head in Community Cohorts
Therapeutics Citations
Paper Citations
- Hu Y, Kirmess KM, Meyer MR, Rabinovici GD, Gatsonis C, Siegel BA, Whitmer RA, Apgar C, Hanna L, Kanekiyo M, Kaplow J, Koyama A, Verbel D, Holubasch MS, Knapik SS, Connor J, Contois JH, Jackson EN, Harpstrite SE, Bateman RJ, Holtzman DM, Verghese PB, Fogelman I, Braunstein JB, Yarasheski KE, West T. Assessment of a Plasma Amyloid Probability Score to Estimate Amyloid Positron Emission Tomography Findings Among Adults With Cognitive Impairment. JAMA Netw Open. 2022 Apr 1;5(4):e228392. PubMed.
- Janelidze S, Bali D, Ashton NJ, Barthélemy NR, Vanbrabant J, Stoops E, Vanmechelen E, He Y, Dolado AO, Triana-Baltzer G, Pontecorvo MJ, Zetterberg H, Kolb H, Vandijck M, Blennow K, Bateman RJ, Hansson O. Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer's disease. Brain. 2022 Sep 10; PubMed.
- Brum WS, Ashton NJ, Simrén J, di Molfetta G, Karikari TK, Benedet AL, Zimmer ER, Lantero-Rodriguez J, Montoliu-Gaya L, Jeromin A, Aarsand AK, Bartlett WA, Calle PF, Coşkun A, Díaz-Garzón J, Jonker N, Zetterberg H, Sandberg S, Carobene A, Blennow K. Biological variation estimates of Alzheimer's disease plasma biomarkers in healthy individuals. Alzheimers Dement. 2024 Feb;20(2):1284-1297. Epub 2023 Nov 20 PubMed.
- Ashton NJ, Brum WS, Di Molfetta G, Benedet AL, Arslan B, Jonatis E, Langhough RE, Cody K, Wilson R, Carlsson CM, Vanmechelen E, Montoliu-Gaya L, Lantero-Rodriguez J, Rahmouni N, Tissot C, Stevenson J, Servaes S, Therriault J, Pascoal T, Lleó A, Alcolea D, Fortea J, Rosa-Neto P, Johnson S, Jeromin A, Blennow K, Zetterberg H. Diagnostic accuracy of the plasma ALZpath pTau217 immunoassay to identify Alzheimer's disease pathology. medRxiv. 2023 Jul 12; PubMed.
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.