Is the ‘Atrophy’ of Immunotherapy Just the Dismantling of Plaque ‘Suburbia’?
Quick Links
The brain shrinkage seen on amyloid immunotherapy remains an unsolved and concerning riddle. At the Alzheimer’s Association International Conference, held July 27 to August 1 in Philadelphia, Nick Fox of University College London offered a possible answer.
- In immunotherapy trials, MRI volume loss does not correlate with degeneration.
- It does correlate with removal of plaques and surrounding material.
- These ‘plaque neighborhoods’ are large enough to explain the excess shrinkage.
While the amount of Aβ removed by antibodies is too small to account for the lost volume, Fox pointed out that amyloid plaques are more than just Aβ. They include a built-up “suburbia” of other proteins, dystrophic neurites, and associated immune cells. All told, this occupies around 6 percent of the cortex. Indeed, some studies have found that the cortex thickens where plaques form. Removal of this material could in fact explain the cortical volume loss, Fox argued.
Greater cortical shrinkage was associated with slower cognitive decline in the Phase 3 lecanemab trial. Rather than calling this volume loss “atrophy,” which implies a degenerative process, Fox proposed calling it “amyloid-removal related pseudo-atrophy,” or ARPA. The data are in press at Lancet Neurology.
Cynthia Lemere of Brigham and Women’s Hospital, Boston, said the term ARPA makes sense, as does the theory behind it. Attendees in Philadelphia were receptive to the idea. Fox’s talk generated the most questions and subsequent buzz of its session.
Maybe a Shrinking Brain Really Can be Good for You
Accelerated volume loss on amyloid immunotherapy was first observed 20 years ago in the AN1792 vaccine trial. At the 2004 AAIC—also held in Philadelphia, and marked by the roving, cheerful, ever-present local “host” John Trojanowski—Fox first reported, to the audience’s consternation, that higher anti-Aβ antibody titers correlated with more brain shrinkage. Even in this discontinued first attempt at immunotherapy, drug response and brain shrinkage also correlated with lower cerebrospinal fluid tau and better cognitive outcomes. This raised the provocative question of whether there are circumstances when a bit of brain shrinkage can be a good thing (see Jul 2004 news coverage and associated comments).
Since then, a small amount of volume loss has become a consistent feature in many immunotherapy trials. This engendered debate over whether it indicates accelerated degeneration, or something good to do perhaps with dampened inflammation. In Philadelphia 2024, Fox argued for the latter. For one, brain shrinkage on immunotherapy is unlikely to represent neuron loss, because it does not correlate with neurodegenerative markers, he said. In the Phase 3 lecanemab trial, CSF total tau went down on drug, as did neurogranin, a marker of synaptic loss. Plasma NfL trended down as well (see related story).

Sign of Neuroprotection? In the lecanemab Phase 3 trial, people on drug (green) had preserved hippocampal volume relative to controls (black). [Courtesy of Bateman et al., CTAD 2022.]
Importantly, hippocampal volume is preserved in many immunotherapy trials. This fits with Fox’s explanation, because there are fewer plaques taking up space in AD hippocampus than cortex to begin with. In the Phase 3 donanemab trial, hippocampal volume loss was the same on drug or placebo (Jul 2023 conference news). In the Phase 3 lecanemab data, the hippocampus shrank less on drug than placebo, with the difference statistically significant (image above). Hippocampal shrinkage is a key indicator of AD, so its preservation could indicate neuroprotection, Fox said.
Further supporting the idea that the whole-brain volume loss on these antibodies is not harmful: It correlated with better cognitive outcomes. At the same degree of cortical shrinkage, people on lecanemab had less functional decline on the CDR-SB and ADCS MCI-ADL than did those on placebo, Fox calculated.
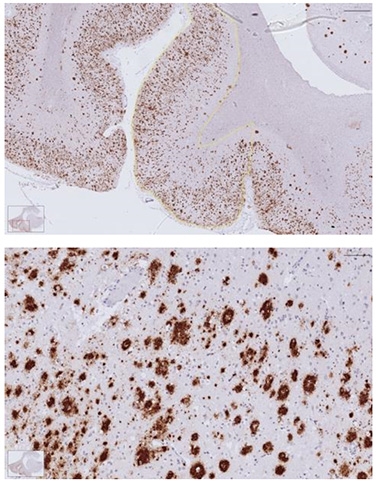
Sprawling Outward? Amyloid plaques and their associated cells and dystrophy occupy around 6 percent of the AD cortex (top) by volume; higher magnification at bottom. [Courtesy of Zane Jaunmuktane, Queen Square Brain Bank, UCL.]
Plaques Crowd the Cortex, Hog Space
Instead, Fox believes the shrinkage is due to clearance of plaque. This had been considered an unlikely explanation for volume loss, in part based on calculations that the AD brain contains a total of 6.5 mg of Aβ (Aug 2016 news; Roberts et al., 2017). Extrapolating from this, scientists have calculated that removing this tiny amount would account for only one-thousandth of the volume change. Fox decided to test this conclusion, noting that in the brain, these protein snarls and their associated cellular changes occupy extensive space.
Postmortem studies that directly measured plaques found they typically took up around 7 percent of the cortical volume (Bussière et al., 2002; Josephs et al., 2008; Clark et al., 2012). More recent papers reported similar numbers, with one multicenter study calculating that diffuse plaques occupy 5 to 8 percent of the cortex, and compact plaques 1 to 2 percent (Chen et al., 2021). Another found plaques accounting for almost 9 percent of frontal cortex, and 6.5 percent of temporal cortex (Abrahamson et al., 2022).
Seven percent of cortex would equate to about 3 percent of whole brain, Fox noted. Given that the excess brain shrinkage in the Phase 3 lecanemab trial was about 0.4 percent, plaque clearance could easily account for it, he said. He noted that the more plaque a person started with at baseline, and the more that was removed, the more their brain shrank compared with that of placebo controls.
The few autopsies of immunotherapy patients published to date show how extensive clearance can be. A woman on aducanumab for 2.5 years had almost no plaques left, while a man on lecanemab for 3.5 years had no diffuse plaques, and sparse neuritic plaques (Plowey et al., 2022; Aug 2022 conference news).
If plaque claims that much space in the brain, shouldn’t the brain get a little bigger as deposits form? Indeed, some studies have reported this. Gael Chételat of Cyceron in Caen, France, found that among cognitively healthy people, those with plaques have slightly bigger brains than those without (Chételat et al., 2010). Likewise, in presymptomatic people with a dominantly inherited AD mutation, the cortex thickens 15 to 20 years before symptom onset (Montal et al., 2021).
Several studies have linked brain expansion in preclinical AD with astrocytosis markers, implying it could be due to inflammatory processes (Vilaplana et al., 2020; Salvadó et al., 2022; Spotorno et al., 2022).
In his AAIC talk, Fox emphasized the role of inflammation as well. Astrocytes and microglia crowd around plaques, and the astrocytic marker GFAP rises as AD progresses. Removing plaque could get rid of these cells, slimming down the cortex. Anti-inflammatory treatments for multiple sclerosis initially shrink the brain, too, he noted.
A recent meta-analysis linked volume loss to ARIA. The more ARIA for a given immunotherapy, the more ventricles expanded on that drug. This implied a corresponding reduction in gray matter, the authors claimed (Apr 2023 news). Fox challenged the view that greater ventricular volume necessarily correlates with gray-matter loss. In the Phase 3 lecanemab trial, ARIA events were associated with 2.6 mL higher ventricular volume, but with no change in whole-brain shrinkage. Possibly, enlarged ventricles are caused by fluid shifts due to inflammatory changes, he suggested.
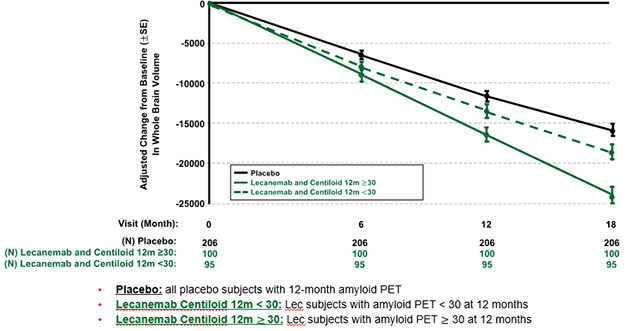
Slowing Shrinkage. In people who had cleared most plaque by 12 months on lecanemab (dotted green line), whole-brain volume loss slowed to about the rate seen on placebo (black), while in those who were still clearing amyloid (solid green), shrinkage remained accelerated. [Courtesy of Esai.]
Does Volume Loss Slow Over Time?
How to distinguish (good) shrinkage due to amyloid removal from (bad) shrinkage due to neurodegeneration? It’s a tricky problem. If amyloid immunotherapy protects the brain and slows neuron loss, then, as plaque gets cleared, accelerated shrinkage should level out. In time, volume loss would be expected to become less on drug than on placebo. Whether this happens is not yet clear, but there are some promising signs, Fox said.
There were hints of this even in the AN1792 trial. A subgroup of participants who responded well to the vaccine lost more brain volume than the placebo group for one year, but had bigger brains than placebo at year two. At AAIC, Fox reported there were hints of this in the 18-month Phase 3 lecanemab trial, too. People who had completely cleared plaque by 12 months lost less gray matter overall than did those who remained amyloid-positive at 12 months. After that point, their brains continued shrinking at nearly the same rate as in the placebo group.
Another finding in support of this hypothesis is the slower shrinkage of hippocampus on lecanemab than placebo. Because the hippocampus contains few plaques, it would be little affected by amyloid clearance, hence its size may more directly reflect neurodegeneration. Eventually, the cortex and whole brain would follow suit, with their rates of decline slowing relative to placebo, Fox said.
Because the lecanemab post-18-month extension is open-label, there are no longer-term comparative data. In addition, brain atrophy accelerates as disease advances, which would also make it hard to see any flattening of the curve (Chan et al., 2003). Fox stressed the importance of longer studies to track brain volume changes over several years on immunotherapy. He also recommended that drug companies make patient-level trial data available to help researchers parse out the relationships between regional plaque content, regional volume changes, and other markers of AD.—Madolyn Bowman Rogers
References
Therapeutics Citations
News Citations
- John Trojanowski, 75, a Giant in the Field of Neuropathology
- Philadelphia: Can a Shrinking Brain Be Good for You?
- Leqembi: The Case for Long-Term Dosing
- Donanemab Data Anchors Upbeat AAIC
- Refining Models of Amyloid Accumulation in Alzheimer’s Disease
- Could Benefit of Plaque Removal Grow in Time?
- What About the Brain Shrinkage Seen with Aβ Removal?
Paper Citations
- Roberts BR, Lind M, Wagen AZ, Rembach A, Frugier T, Li QX, Ryan TM, McLean CA, Doecke JD, Rowe CC, Villemagne VL, Masters CL. Biochemically-defined pools of amyloid-β in sporadic Alzheimer's disease: correlation with amyloid PET. Brain. 2017 May 1;140(5):1486-1498. PubMed.
- Bussière T, Friend PD, Sadeghi N, Wicinski B, Lin GI, Bouras C, Giannakopoulos P, Robakis NK, Morrison JH, Perl DP, Hof PR. Stereologic assessment of the total cortical volume occupied by amyloid deposits and its relationship with cognitive status in aging and Alzheimer's disease. Neuroscience. 2002;112(1):75-91. PubMed.
- Josephs KA, Whitwell JL, Ahmed Z, Shiung MM, Weigand SD, Knopman DS, Boeve BF, Parisi JE, Petersen RC, Dickson DW, Jack CR. Beta-amyloid burden is not associated with rates of brain atrophy. Ann Neurol. 2008 Feb;63(2):204-12. PubMed.
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, Fleisher AS, Reiman EM, Sabbagh MN, Sadowsky CH, Schneider JA, Arora A, Carpenter AP, Flitter ML, Joshi AD, Krautkramer MJ, Lu M, Mintun MA, Skovronsky DM, . Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012 Aug;11(8):669-78. PubMed.
- Chen CD, Joseph-Mathurin N, Sinha N, Zhou A, Li Y, Friedrichsen K, McCullough A, Franklin EE, Hornbeck R, Gordon B, Sharma V, Cruchaga C, Goate A, Karch C, McDade E, Xiong C, Bateman RJ, Ghetti B, Ringman JM, Chhatwal J, Masters CL, McLean C, Lashley T, Su Y, Koeppe R, Jack C, Klunk WE, Morris JC, Perrin RJ, Cairns NJ, Benzinger TL. Comparing amyloid-β plaque burden with antemortem PiB PET in autosomal dominant and late-onset Alzheimer disease. Acta Neuropathol. 2021 Oct;142(4):689-706. Epub 2021 Jul 28 PubMed.
- Abrahamson EE, Kofler JK, Becker CR, Price JC, Newell KL, Ghetti B, Murrell JR, McLean CA, Lopez OL, Mathis CA, Klunk WE, Villemagne VL, Ikonomovic MD. 11C-PiB PET can underestimate brain amyloid-β burden when cotton wool plaques are numerous. Brain. 2022 Jun 30;145(6):2161-2176. PubMed.
- Plowey ED, Bussiere T, Rajagovindan R, Sebalusky J, Hamann S, von Hehn C, Castrillo-Viguera C, Sandrock A, Budd Haeberlein S, van Dyck CH, Huttner A. Alzheimer disease neuropathology in a patient previously treated with aducanumab. Acta Neuropathol. 2022 Jul;144(1):143-153. Epub 2022 May 17 PubMed.
- Chételat G, Villemagne VL, Pike KE, Baron JC, Bourgeat P, Jones G, Faux NG, Ellis KA, Salvado O, Szoeke C, Martins RN, Ames D, Masters CL, Rowe CC. Larger temporal volume in elderly with high versus low beta-amyloid deposition. Brain. 2010 Nov;133(11):3349-58. PubMed.
- Montal V, Vilaplana E, Pegueroles J, Bejanin A, Alcolea D, Carmona-Iragui M, Clarimón J, Levin J, Cruchaga C, Graff-Radford NR, Noble JM, Lee JH, Allegri R, Karch CM, Laske C, Schofield PR, Salloway S, Ances B, Benzinger T, McDale E, Bateman R, Blesa R, Sánchez-Valle R, Lleó A, Fortea J, Dominantly Inherited Alzheimer Network (DIAN). Biphasic cortical macro- and microstructural changes in autosomal dominant Alzheimer's disease. Alzheimers Dement. 2021 Apr;17(4):618-628. Epub 2020 Nov 16 PubMed.
- Vilaplana E, Rodriguez-Vieitez E, Ferreira D, Montal V, Almkvist O, Wall A, Lleó A, Westman E, Graff C, Fortea J, Nordberg A. Cortical microstructural correlates of astrocytosis in autosomal-dominant Alzheimer disease. Neurology. 2020 May 12;94(19):e2026-e2036. Epub 2020 Apr 14 PubMed.
- Salvadó G, Shekari M, Falcon C, Operto G, Milà-Alomà M, Sánchez-Benavides G, Cacciaglia R, Arenaza-Urquijo E, Niñerola-Baizán A, Perissinotti A, Minguillon C, Fauria K, Kollmorgen G, Suridjan I, Molinuevo JL, Zetterberg H, Blennow K, Suárez-Calvet M, Gispert JD, ALFA Study. Brain alterations in the early Alzheimer's continuum with amyloid-β, tau, glial and neurodegeneration CSF markers. Brain Commun. 2022;4(3):fcac134. Epub 2022 May 24 PubMed.
- Spotorno N, Strandberg O, Vis G, Stomrud E, Nilsson M, Hansson O. Measures of cortical microstructure are linked to amyloid pathology in Alzheimer's disease. Brain. 2022 Sep 21; PubMed.
- Chan D, Janssen JC, Whitwell JL, Watt HC, Jenkins R, Frost C, Rossor MN, Fox NC. Change in rates of cerebral atrophy over time in early-onset Alzheimer's disease: longitudinal MRI study. Lancet. 2003 Oct 4;362(9390):1121-2. PubMed.
Further Reading
Follow-On Reading
Papers
- Belder CR, Boche D, Nicoll JA, Jaunmuktane Z, Zetterberg H, Schott JM, Barkhof F, Fox NC. Brain volume change following anti-amyloid β immunotherapy for Alzheimer's disease: amyloid-removal-related pseudo-atrophy. Lancet Neurol. 2024 Oct;23(10):1025-1034. PubMed.
- Hyman BT. Amyloid removal and the appearance of brain volume loss. Lancet Neurol. 2024 Oct;23(10):957-958. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.